Abstract
Ferroptosis, a unique modality of cell death with mechanistic and morphological differences from other cell death modes, plays a pivotal role in regulating tumorigenesis and offers a new opportunity for modulating anticancer drug resistance. Aberrant epigenetic modifications and posttranslational modifications (PTMs) promote anticancer drug resistance, cancer progression, and metastasis. Accumulating studies indicate that epigenetic modifications can transcriptionally and translationally determine cancer cell vulnerability to ferroptosis and that ferroptosis functions as a driver in nervous system diseases (NSDs), cardiovascular diseases (CVDs), liver diseases, lung diseases, and kidney diseases. In this review, we first summarize the core molecular mechanisms of ferroptosis. Then, the roles of epigenetic processes, including histone PTMs, DNA methylation, and noncoding RNA regulation and PTMs, such as phosphorylation, ubiquitination, SUMOylation, acetylation, methylation, and ADP-ribosylation, are concisely discussed. The roles of epigenetic modifications and PTMs in ferroptosis regulation in the genesis of diseases, including cancers, NSD, CVDs, liver diseases, lung diseases, and kidney diseases, as well as the application of epigenetic and PTM modulators in the therapy of these diseases, are then discussed in detail. Elucidating the mechanisms of ferroptosis regulation mediated by epigenetic modifications and PTMs in cancer and other diseases will facilitate the development of promising combination therapeutic regimens containing epigenetic or PTM-targeting agents and ferroptosis inducers that can be used to overcome chemotherapeutic resistance in cancer and could be used to prevent other diseases. In addition, these mechanisms highlight potential therapeutic approaches to overcome chemoresistance in cancer or halt the genesis of other diseases.
Similar content being viewed by others
Introduction
Ferroptosis, a new form of regulated cell death (RCD), is driven by iron-dependent lipid peroxidation (LPO) of polyunsaturated fatty acid-containing phospholipids (PUFA-PLs) in cellular membranes.1,2,3,4 Ferroptosis was first reported in 2012 and was found to be induced by erastin, an oncogenic RAS-selective lethal chemical.4 Ferroptosis was officially identified as a non-apoptotic RCD triggered by intracellular iron perturbations and oxidative stress.5 A imbalance between ferroptosis defense and systems dictates the execution and induction of ferroptosis.6 Many metabolic pathways and degradation pathways orchestrate the complex response to ferroptosis by indirectly or directly regulating LPO or iron accumulation.7 The metabolic pathways include pathways related to lipid, iron, and amino acid metabolism, and the degradation pathways include pathways such as the ubiquitin–proteasome system (UPS) and macroautophagy/autophagy.
Accumulating evidence suggests that ferroptosis is precisely regulated at multiple levels that include protein posttranslational modifications (PTMs) and epigenetic modifications.8 Epigenetic modification that includes DNA methylation, histone modification, and noncoding RNA (ncRNA) regulation is a dynamic and reversible process, which regulates gene expression without changing the DNA sequence.9,10,11 PTMs covalently or enzymaticly modify or introduce functional groups to dynamically modulate protein localization, activity, and molecular interactions.12,13 PTMs include phosphorylation, ubiquitination, SUMOylation, acetylation, among others.13 Aberrant epigenetic modifications and PTMs dynamically drive abnormal transcription or translation processes to promote anticancer drug resistance, cancer progression, metastasis, etc. The epigenetic modifications regulate the expression levels of ferroptosis-related genes, consequently determining the vulnerability of cancer cells to ferroptosis at both the transcriptional and translational levels.8,14,15,16 Moreover, emerging evidence has revealed the roles of epigenetic modifications and PTMs in the regulation of ferroptosis in NSDs, CVDs, liver diseases, lung diseases, and kidney diseases.17
Dysregulated ferroptosis is increasingly recognized as a significant contributor to the pathogenesis of diseases, including cancers,18 nervous system diseases (NSDs),19,20,21,22,23 cardiovascular diseases (CVDs),24,25,26,27,28 liver diseases,29,30 lung diseases,31,32,33,34, and kidney diseases.35 Recently, ferroptosis has been recognized to play an important role in halting tumor growth.36 In the last decade, accumulating evidence has revealed the role of ferroptosis in tumor growth suppression and shown that ferroptosis induction partially mediates the tumor-suppressive effects of chemotherapy.3,37 Ferroptosis determines the efficacy of chemotherapy, immunotherapy, and radiotherapy, and thus, combination treatments with ferroptosis inducers could boost the efficacy of those therapies.38,39 Accumulating studies have shown that pharmacologically modulating ferroptosis may be a therapeutic approach for NSDs, CVDs, liver diseases, lung diseases, and kidney diseases.19,25,40,41,42,43,44,45
In this review, we first summarize the core molecular mechanisms of ferroptosis. Then, the role of epigenetic processes, including histone PTMs, DNA methylation, and ncRNA regulation, are concisely discussed. This discussion is followed by a detailed description of the roles of epigenetic regulation of ferroptosis in the genesis of diseases, including NSD, CVD, liver diseases, lung diseases, and kidney diseases, as well as the application of epigenetic modulators in the treatment of these diseases. Elucidating the epigenetic regulatory mechanisms of ferroptosis in cancer and other diseases will accelerate the development of promising combination therapeutic regimens containing epigenetic agents and ferroptosis inducers that can be used to overcome chemotherapeutic resistance in cancer and could be used to prevent other diseases. In addition, these mechanisms and highlight promising therapeutic approaches that may be used to overcome chemotherapy drug resistance in cancer or halt the genesis of other diseases.
Molecular mechanisms of ferroptosis
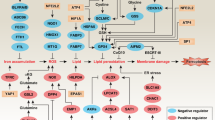
The term ferroptosis was coined by the Stockwell laboratory in 2012 based on the three major research areas that provided a foundational understanding of ferroptosis.: the control of ROS,46,47 the mechanisms of amino acid and lipid metabolism,48,49,50 and the regulation of iron2 (Fig. 1). Iron accumulation and LPO trigger ferroptosis, resulting in plasma membrane rupture.51 The initiation of ferroptosis requires two key signals, namely, the accumulation of free iron and the inhibition of defense systems, mainly the solute carrier family seven members 11–glutathione–glutathione peroxidase 4 (SLC7A11–GSH–GPX4) system.52 The activation of ferroptosis indicates a delicate imbalance between ferroptosis-promoting factors and defense systems. When the former factors significantly override the latter antioxidant defense systems, lethal accumulation of lipid peroxides on cellular membranes leads to membrane rupture and results in ferroptosis-related cell death3,6 (Fig. 2). Currently, the main ferroptosis defense systems constitute the SLC7A11–GSH–GPX4 system,6,53 the GTP cyclohydrolase 1–tetrahydrobiopterin (GCH1–BH4) system,54,55 the ferroptosis suppressor protein 1–ubiquinol (FSP1–CoQH2) system,56,57 the dihydroorotate dehydrogenase–dihydroubiquinone (DHODH–CoQH2) system,58 and the O-acyltransferase domain containing 1/2–monounsaturated fatty acids (MBOAT1/2–MUFA) system.59 Many key components of the ferroptosis pathway, e.g., the principal proteins and enzymes engaged in the induction and inhibition of ferroptosis, are transcriptionally controlled by NF-E2 p45-related factor 2 (Nrf2), the transcription factor encoded by NFE2L2.60,61,62,63,64 Nrf2 and Kelch-like ECH-associated protein 1 (KEAP1), which is the principal negative regulator of Nrf2 and an E3 ligase adaptor, are critical for maintaining metabolic, redox and protein homeostasis.65,66 Nrf2 is involved in regulating the transcription of enzymes responsible for GSH biosynthesis, such as glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit, glutathione disulfide reductase, and GSH synthetase (GSS), which support GPX4-mediated suppression of ferroptosis by increasing and maintaining the GSH level. Other downstream targets of Nrf2 include SLC7A11, GPX4, and NAD(P)H-quinone oxidoreductase 1, as well as iron metabolism proteins, such as ferritin light chain (FTL), ferritin heavy chain 1 (FTH1), ferroportin-1 (FPN1) and heme oxygenase-1 (HO-1), all of which are directly relevant to ferroptosis.60,61,62,63,64,67,68,69
Core mechanisms of ferroptosis. The core of ferroptosis initiation is iron-dependent lipid peroxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids (PUFA-PLs). When the ferroptosis-promoting factors (or Ferroptosis prerequisites) exceeding the buffering capability of cellular antioxidant systems (or ferroptosis defense systems), lethal accumulation of lipid peroxides on cellular membranes lead to membrane rupture, resulting in ferroptosis-related cell death. The ferroptosis-promoting factors consist of PUFA-PL synthesis and peroxidation, iron metabolism among others. Cells have evolved at least four ferroptosis defense systems,which includes GPX4/xCT system, the FSP1/CoQH2 system, the DHODH /CoQH2 system, and the GCH1/BH4 system, with different subcellular localizations to detoxify lipid peroxides and thus protect cells against ferroptosis. The cytosolic GPX4 (GPX4cyto) cooperates with FSP1 on the plasma membrane (and other non-mitochondrial membranes) and mitochondrial GPX4 (GPX4mito) cooperates with DHODH in the mitochondria to neutralize lipid peroxides. ACSL4 and LPCAT3 mediate the synthesis of PUFA-PLs, which are susceptible to LPO through both non-enzymatic and enzymatic mechanisms. Iron initiates the non-enzymatic Fenton reaction and acts as an essential cofactor for ALOXs and POR, which promote LPO. When ferroptosis-promoting factors significantly exceed the detoxification capabilities of ferroptosis defense systems, an excessive and lethal accumulation of lipid peroxides on cellular membranes result in membrane rupture and trigger ferroptosis-mediated cell death
Ferroptosis-promoting factors
PUFA-PL synthesis and peroxidation
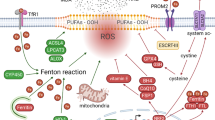
The core mechanism of ferroptosis is membrane LPO, a radical-induced chain reaction consisting of a series of chemical reactions between molecular oxygen (O2), oxidizable lipids, and iron, leading to the incorporation of O2 into lipids.70,71 Because of their susceptibility to peroxidation, PUFA-PLs are the substrates for LPO during ferroptosis.70,72 The critical mediators of the synthesis of PUFA-PLs include acyl-coenzyme A (CoA) synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3).73,74 ACSL4 ligates the free long-chain PUFAs adrenic acid (AdA) and arachidonic acid (AA) to CoA to generate AA-CoA and ADA-CoA (i.e., PUFA-CoAs), respectively.74,75 Subsequently, these PUFA-CoAs are re-esterified and incorporated into PLs by LPCAT3 to produce PUFA-PLs (such as AA-phosphatidylethanolamine [PE] and ADA-PE).73,75,76 PKCβII-mediated phosphorylation of ACSL4 can further activate ACSL4.77 Acetyl-CoA carboxylase mediates the synthesis of PUFAs from the basic building block acetyl-CoA.72 Nonenzymatic autoxidation through the iron-mediated Fenton reaction is the primary driver of LPO of PUFA-PLs.70,78,79 The enzymatic reactions mediated by arachidonate lipoxygenase (ALOX) or Cytochrome P450 oxidoreductase are also involved in facilitating LPO.80,81,82,83,84 final step of ferroptosis is the formation of pores in plasma or organelle membranes driven by LPO or its secondary products (4-hydroxynonenal and malondialdehyde), which eventually results in cell death. During recent decades, the involvement of ferroptosis in diseases has attracted great interest, not only in the cancer research community.85,86
Iron homeostasis
Free iron is involved in the core mechanism of ferroptosis in at least two different ways.: inducing accumulation of lethal lipid peroxides and initiating ferroptosis by catalyzing the nonenzymatic Fenton reaction for direct peroxidation of PUFA-PLs70,79 or functioning as an essential cofactor for POR and ALOX, both of which are enzymes that participate in LPO.52,80,81,87 Mammalian cells contain a relatively stable pool of intracellular iron—the labile iron pool (LIP)—and maintain this pool by orchestrating the regulation of iron uptake, utilization, storage (ferritin: FTH1/FTL) and export (FPN, the iron export transporter).88,89 Deregulation of iron metabolism processes can suppress or promote ferroptosis as a result of a decrease or increase in intracellular LIP, respectively. Intracellular iron is mostly stored in the ferritin protein complex, which is composed of 24 subunits of FTL and FTH1.90 Poly rC-binding protein 1 binds to and incorporates ferrous iron (Fe2+) into ferritin, and this Fe2+ is further oxidized to ferric iron (Fe3+) by FTH1 inside the ferritin cage, resulting in inert deposits of Fe3+ that are unavailable for intracellular use or ROS production.91 Ferritinophagy, a nuclear receptor coactivator 4 (NCOA4)-mediated autophagy-like process, can degrade ferritin, release iron stored in ferritin into the LIP and facilitate the availability of iron in cells, thereby boosting LPO-driven ferroptosis.72,92,93,94,95 Through its function as a selective autophagy receptor, NCOA4 transports intracellular ferritin to autophagic lysosomes and releases free iron through binding FTH1.96 Inhibition of cytosolic glutamate oxaloacetate transaminase 1, which enhances ferritinophagy, can increase the LIP and promote ferroptosis.97 Conversely, inhibition of ferritinophagy mediated by NCOA4 decreases the LIP and suppresses ferroptosis.93,94
Ferroptosis defense systems
SLC7A11–GSH–GPX4 axis
There are four antiferroptosis defense systems (or cellular antioxidant systems) that directly neutralize lipid peroxides with distinctive subcellular localizations. Related to the metabolism of amino acids, the SLC7A11–GSH–GPX4 axis is a well-defined and major antiferroptosis defense system.6,53 SLC7A11 (also named xCT) and solute carrier family 3 member 2 constitute system Xc−.98,99 xCT is a transporter subunit and mediates the antiporter activity of system Xc− through the import of extracellular cystine and export of intracellular glutamate.98,100 Through a reduction reaction mediated by nicotinamide adenine dinucleotide phosphate (NADPH) consumption in the cytosol, extracellular cystine taken up via SLC7A11 is rapidly reduced to cysteine, which then serves as the rate-limiting precursor for GSH biosynthesis.99 GSH is the major cofactor for GPX4-mediated detoxification of lipid peroxides.99 Inhibition of SLC7A11 activity or depletion of cystine promotes ferroptosis in various cancer cells.99 GPX4 is a member of the GPX protein family with enzymatic lipid repair activity,101,102 and it has been identified as a key ferroptosis inhibitor.4,103,104 GPX4 can promote the production of nontoxic lipid PL alcohols from PL hydroperoxides (L-OOH) and simultaneously oxidize two GSH molecules to yield oxidized GSH (GSSG).46,105 Accumulating studies have revealed the critical role of GPX4 in inhibiting ferroptosis through its genetic or pharmacological manipulation.106,107 GPX4 is regulated by epigenetic modifications and PTMs.108 Small molecule inhibitors of GPX4 could be optimized for use as anticancer agents.109
The FSP1–CoQH2 system
Several nonperoxidase mechanisms function in parallel with GPX4 to inhibit LPO and ferroptosis. FSP1, localized on the plasma membrane, is also known as apoptosis-inducing factor mitochondria-associated 2. In 2019, FSP1 was identified as the second main protein inhibiting ferroptosis independent of GPX4 through the production of coenzyme Q10 (CoQ10, also known as ubiquinone), reduced forms of endogenous electron carriers and vitamin K, all of which possess significant antioxidant (RTA) activity.56,57,110 FSP1 functions as an NAD(P)H-dependent oxidoreductase to reduce CoQ10 to regenerate CoQ10-H2, the reduced form of CoQ10, which can trap lipid peroxyl radicals to hinder LPO and halt ferroptosis.56,57 FSP1 also inhibits ferroptosis independently of its oxidoreductase function by activating ESCRT-III-dependent membrane repair, which halts ferroptosis.111,112 Small molecule inhibitors of FSP1 could also be optimized as anticancer agents.57
The GCH1–BH4 system
A study in 2020 revealed that the GCH1–BH4 system is another critical GPX4-independent inhibitor of ferroptosis that acts by suppressing LPO.54,55 GCH1, which mediates the rate-limiting reaction generating the endogenous metabolite BH4, was discovered as a suppressor by Kraft54 and as an enhancer of ferroptosis by Birsoy in 2020.55 As a different RTA, BH4 can be regenerated following its RTA reactions by dihydrofolate reductase (DHFR). Inhibition of DHFR synergizes with inhibition of GPX4 to induce ferroptosis.55 Moreover, BH4 enhances CoQ10 synthesis by converting phenylalanine into tyrosine, which can be further converted to 4-OH-benzoate, the precursor of CoQ10.54 GCH1-mediated BH4 synthesis reprograms lipid metabolism and inhibits ferroptosis by selectively preventing two polyunsaturated fatty acyl tails from depleting PLs.54
The DHODH–CoQH2 system
A newly identified GPX4-independent mitochondria-localized ferroptosis defense system, the DHODH–CoQH2 system can compensate for GPX4 loss and detoxify mitochondrial lipid peroxides.58 DHODH is localized to the inner mitochondrial membrane, where it catalyzes de novo pyrimidine synthesis through which CoQ10 can be reduced to CoQH2 at the rate-limiting fourth step. The function of CoQH2 is analogous to that of FSP1 in extra-mitochondrial membranes.58 After acute inactivation of GPX4, DHODH-mediated flux is significantly increased, leading to increased generation of CoQH2, which neutralizes LPO and inhibits ferroptosis in mitochondria.58 Inactivation of both mitochondrial DHODH and GPX4 causes robust ferroptosis by unleashing potent LPO reactions in mitochondria.6 Low expression of DHODH or high expression of GCH1 renders cells more sensitive or resistant to ferroptosis, respectively.
MBOAT1/2–MUFA system
The MBOAT1/2-PE-MUFA system is a newly identified ferroptosis defense mechanism independent of GPX4 and FSP1.59 Jiang and colleagues identified new PL-modifying enzymes, MBOAT1 and MBOAT2, which function as ferroptosis suppressors.59 By functioning as a lyso-PL acyltransferase (LPLAT), membrane-bound MBOAT2 inhibits ferroptosis by selectively incorporating MUFAs into lysophosphatidylethanolamine (lyso-PE), thereby correspondingly increasing the abundance of cellular PE-MUFAs and decreasing the abundance of cellular PE-PUFAs. PE-PUFAs are the preferred substrate for LPO and determine ferroptosis sensitivity.73,74 The sex hormone receptors, i.e., the estrogen receptor (ER) and androgen receptor (AR), directly transcriptionally upregulate MBOAT1 and MBOAT2, respectively. AR or ER antagonists boost the antitumor activity of ferroptosis inducers in AR+ prostate cancers and ER+ breast cancers with or without resistance to single-agent hormone therapies.59
Epigenetic and posttranslational modifications
Epigenetic modification, a dynamic and reversible process, regulates gene expression without changing the DNA sequence.9,10 There are four major mechanisms of epigenetic regulation: DNA methylation, chromatin structure regulation, histone PTM, and ncRNA regulation.9,10,113 The common well-studied epigenetic regulatory mechanisms are DNA methylation, histone modification, and ncRNA regulation.11 The histone subunit in the nucleosome contains a characteristic tail possessing specific amino acids for covalent PTMs, such as ubiquitination, phosphorylation, methylation, acetylation, SUMOylation, acylation, glycosylation, hydroxylation, serotonylation, glycation, and ADP-ribosylation.114,115,116,117 Epigenetic regulation of gene expression is mediated by various classes of proteins, most of which have enzymatic activities. Four classes of epigenetic regulators, i.e., “writers”, “erasers”, “readers”, and “remodelers”, constitute the molecular component of the epigenetic regulators of DNA and histone modifications and chromatin structure.113,118 The writers and erasers add and remove epigenetic marks, respectively. The readers recognize specific epigenetic marks to mediate downstream effects, while the remodelers modulate the chromatin state.10 Approximately 1000 epigenetic regulators form one of the largest protein groups in mammals. Cancer develops as a result of progressive accumulation of cell-intrinsic genetic and epigenetic changes, which are key characteristics of most cancers.119,120 Epigenetic mechanisms regulate cancer biology in multiple ways, including driving tumorigenesis and invasion and modulating the immune response. Furthermore, modulation of the epigenome exposes cancer cells to immune-mediated attack, increasing cancer cell sensitivity to immunotherapy in various solid tumors.121,122 As covalent or enzymatic modifications of synthesized proteins, PTMs modify or introduce functional groups, such as phosphoryl, methyl, acetyl and glycosyl groups, to dynamically modulate protein localization, activity and molecular interactions.12,13 PTMs include phosphorylation, ubiquitination, SUMOylation, acetylation, methylation, ADP-ribosylation, palmitoylation, neddylation, glycosylation, prenylation, cholesterylation, myristoylation, glutathionylation, sulfhydration, citrullination, S-nitrosylation, and several novel PTMs.13 PTMs are usually reversible. Epigenetic modifications and PTMs are strongly correlated with the occurrence and genesis of many diseases. Epigenetic modifications and PTMs transcriptionally and posttranscriptionally regulate gene expression, respectively, and posttranscriptionally modulate protein activity, function and degradation.123 These modifications are required for the maintenance of tissue-specific expression of genes and proteins and for normal cellular development. Dysregulation of epigenetic modifications and PTMs causes aberrant expression of gene and protein signatures and transformation into malignant phenotypes, which induces disease onset and progression.123,124,125 Accumulating evidence has revealed that dysregulated epigenetic regulation contributes to tumor drug resistance, NSDs, CVDs, liver diseases, lung diseases, and kidney diseases.
Epigenetic and posttranslational modifications regulating ferroptosis in diseases
Epigenetic and posttranslational modifications regulating ferroptosis in cancer
Ubiquitination-mediated regulation of ferroptosis in cancer
Ubiquitination is a key and highly conserved PTM and plays a vital role in modulating the degradation of various protein substrates.126,127 Deubiquitinases (DUBs) can remove ubiquitin chains to reverse ubiquitination, leading to termination of ubiquitination and preservation of substrate protein expression.127 The interaction between ubiquitination by ubiquitinases and DUBs plays an important role in controlling almost all aspects of biological activities. Emerging studies have revealed that deubiquitination/ubiquitination are involved in regulating ferroptosis in cancer. Specific regulators can modulate ferroptosis by regulating the ubiquitination of ferroptosis-related factors (Table 1 and Fig. 3).
Posttranslational modification of ferroptosis by ubiquitination in cancer. ALL acute lymphoblastic leukemia; BAP1 tumor suppressor BRCA1-associated protein 1, BC breast cancer, CCA cholangiocarcinoma, CISD2 CDGSH iron sulfur domain 2, ccRCC clear cell renal cell carcinoma, CRC colorectal cancer, ESCC esophageal squamous cell carcinoma, GBC gallbladder cancer, GBM glioblastoma, GC gastric cancer, HCC hepatocellular carcinoma, HNRNPA2B1 heterogeneous nuclear ribonucleoprotein A2/B1, OC ovarian cancer, QSOX1 quiescin sulfhydryl oxidase 1, TEAD4 TEA domain family member 4, USP ubiquitin specific peptidase, RND1 Rho family GTPase 1, SOCS2 suppressor of cytokine signaling 2, βTrCP beta-transducin repeat containing E3 ubiqutin protein ligase
Hepatocellular carcinoma (HCC): Suppressor of cytokine signaling 2-mediated ubiquitination of SLC7A11 enhances ferroptosis and radiosensitization in HCC.128 PCDHB14 functions as a novel tumor suppressor by enhancing ferroptosis through promotion of RNF182-dependent ubiquitination of p65 and blockade of its binding to the promoter of SLC7A11, thereby downregulating SLC7A11 in HCC.129 P53 binds to PCDHB14 to induce its expression.129 Quiescin sulfhydryl oxidase 1 (QSOX1) promotes ferroptosis induced by sorafenib in HCC by driving ubiquitin-mediated degradation of EGFR, leading to suppression of Nrf2 activation, suggesting that QSOX1 is an inducer of ferroptosis.130 Tribbles homolog 2 (TRIB2) functions as one of the key molecules that stabilizes GPX4 and attenuates oxidative stress-induced cell damage.131 A recent study revealed that TRIB2 inhibits and desensitizes ferroptosis through βTrCP-mediated ubiquitination of TfRC, thereby leading to a decline in the LIP in liver cancer cells.132 The largest RNA-binding protein, high-density lipoprotein-binding protein (HDLBP), is an important transporter that protects cells against cholesterol overaccumulation. Elevated expression of HDLBP stabilizes lncFAL to decrease ferroptosis vulnerability by diminishing TRIM69-mediated FSP1 degradation in HCC cells.133 Inhibition of FSP1 enhances the antitumor activity of ferroptosis inducers, supporting the potential utility of targeting FSP1 as a therapeutic approach for HCC patients with high HDLBP or lncFAL expression.133
Gastric cancer (GC): Erastin and RSL3 upregulate the cation channel transient receptor potential melastatin-2 (TRPM2) in GC cell lines. TRPM2 knockdown induces ferroptosis in GC cells, as evidenced by the reductions in the GSH content and GPX activity and increased concentrations of Fe2+, ROS and lipid peroxides. Silencing TRPM2 increases the sensitivity of GC cells to RSL3- and erastin-induced ferroptosis by destabilizing the HIF-1α and Nrf2 proteins, suggesting that TRPM2 functions as a negative regulator of erastin- and RSL3-induced ferroptosis134 and indicating that the combination of TRPM2-targeted drugs with chemotherapeutics potentiates the effectiveness of treatment and improves the outcome of patients. Increased expression of the long noncoding RNA (lncRNA) BDNF-AS inhibits ferroptosis by recruiting WDR5 to transcriptionally upregulate FBXW7, thereby mediating the ubiquitin-dependent degradation of VDAC3 and promoting the progression of GC.135 Increased expression of CST1 promotes the progression and metastasis of GC via inhibition of ferroptosis by reducing GPX4 ubiquitination and degradation by recruiting OTUB1.136 Paclitaxel and cisplatin (CDDP) increase the secretion of cancer-associated fibroblast-derived miR-522 by activating the USP7/hnRNPA1 axis, thereby suppressing ALOX15 expression and ferroptosis and ultimately leading to reduced chemosensitivity.137
Colorectal cancer (CRC): Increased expression of LINC00239 is associated with poorer prognosis in patients with CRC.138 Overexpression of LINC00239 inhibits erastin- and RSL3-mediated antitumor activity by inhibiting ferroptosis. LINC00239 interacts with and binds to the Kelch domain of Keap1, thereby inhibiting the ubiquitination of Nrf2 to stabilize it, suggesting that LINC00239 functions as an oncogene by inhibiting ferroptosis by binding to Keap1 and stabilizing Nrf2 in CRC cells.138 Loss of ribonucleotide reductase subunit M1 (RRM1) destabilizes p53 and increases the sensitivity of different types of cancer cells to ferroptosis by inhibiting the expression of GPX4. Silencing RRM1 promotes the interaction of MDM2 and P53 while decreasing the binding of USP11 to p53, thereby stimulating the ubiquitination of p53, in turn leading to decreased expression of p21, which eventually induces ferroptosis through inhibition of GPX4 and results in cancer cell death.139
Cholangiocarcinoma (CCA): FBXO31 functions as a tumor suppressor, and its expression increases the sensitivity of stem cell-like cells to cisplatin by enhancing ferroptosis through promotion of the proteasomal degradation of GPX4 in CCA cells.140 The expression of a component of the linear ubiquitin chain activation complex, shank-associated RH domain interacting protein (SHARPIN), was found to be increased, promoting cell proliferation through ferroptosis inhibition mediated by promoting the ubiquitination and degradation of P53, thereby upregulating SLC7A11/GPX in CCA cells.141 Blocking SHARPIN-mediated inhibition of ferroptosis via the P53/SLC7A11/GPX4 axis and targeting SHARPIN might be promising treatment approaches for CCA.
Non-small cell lung carcinoma (NSCLC): Inducing ferroptosis is a good treatment approach for LUAD patients with late-stage and/or therapy-resistant tumors. Erastin promotes the nuclear translocation of USP7 by increasing its interaction with p53, which erases the monoubiquitination of lysine 120 on histone H2B (H2Bub1) in the SLC7A11 gene regulatory region and inactivates SCL7A11 expression, eventually leading to ferroptosis in NSCLC cells.142 TRIM6 functions as an oncogene by promoting SLC1A5 ubiquitination and degradation, thereby inhibiting glutamine import, glutaminolysis, LPO, and ferroptosis.143 USP35 is upregulated in NSCLC. USP35 knockdown promotes ferroptosis and increases the sensitivity of lung cancer cells to paclitaxel and cisplatin.144 Conversely, overexpression of USP35 facilitates lung cancer cell growth and tumor progression by reducing Erastin/RSL3-triggered ferroptosis. USP35 directly interacts with the FPN protein to maintain its stability, suggesting that USP35 functions as an oncogene by inhibiting ferroptosis through stabilization of FPN.144 USP11 deubiquitinates and stabilizes Nrf2. Elevated USP11 expression promotes cancer cell proliferation by inhibiting ferroptosis through deubiquitination and stabilization of Nrf2 in NSCLC cells.145
Glioblastoma: Overexpression of TRIM7 inhibits NCOA4-mediated ferritinophagy and ferroptosis through directly binding and ubiquitinating NCOA4 in human glioblastoma cells. Ablation of TRIM7 increases the sensitivity of human glioblastoma cells to temozolomide, suggesting that TRIM7 functions as a negative regulator of ferroptosis.146 Downregulated expression of Rho family GTPase 1 (RND1) predicts a better prognosis in patients with glioblastoma multiforme (GBM). RND1 induces ferroptosis by interacting with and deubiquitinating P53, thereby inhibiting SLC7A11 in GBM cells.147 Circular RNAs (circRNAs) regulate ferroptosis through several mechanisms in GBM. Downregulation of circLRFN5 promotes malignancy by inhibiting ferroptosis in GBM cells. CircLRFN5 binds to the paired related homeobox 2 (PRRX2) protein and promotes its ubiquitin-mediated degradation, thereby transcriptionally upregulating the expression of the ferroptosis suppressor GCH1 in glioma stem cells (GSCs), leading to ferroptosis induction.148
Clear cell renal cell carcinoma (ccRCC): As a tumor suppressor, the H2A DUB BRCA1-associated protein 1 (BAP1) suppresses tumorigenesis by inducing ferroptosis through suppression of SLC7A11 in ccRCC. BAP1, which encodes a nuclear DUB to reduce histone 2A ubiquitination (H2Aub) on chromatin, reduces the H2Aub level in the SLC7A11 promoter and suppresses SLC7A11 expression in a deubiquitination-dependent manner, leading to inhibition of cystine uptake, LPO and ferroptosis.149 BAP1 inhibits the progression of tumors partially by inducing ferroptosis through suppression of SLC7A11 expression, and cancer-associated BAP1 mutants lose their ability to suppress SLC7A11 and promote ferroptosis. BAP1 promotes Erastin-induced ferroptosis by repressing SLC7A11 expression. BAP1 and PRC1 (a major H2Aub ligase) coordinately suppress SLC7A11 expression by regulating the level of H2Aub in the SLC7A11 promoter.150
Gynecologic neoplasms: An increased expression level of estrogen receptor 1 (ESR1) promotes cancer by inhibiting ferroptosis in breast cancer cells through NEDD4L-mediated ubiquitination and degradation of CD71.150 Silencing ESR1 significantly promotes ionizing radiation-mediated ferroptosis and increases the CD71 protein level.150 The results suggest that in breast cancer, ESR1 is an inhibitor of ferroptosis, while CD71 is an inducer of ferroptosis.150 Methylenetetrahydrofolate reductase (MTHFR), a key enzyme for folic acid metabolism, inhibits TRC8-mediated HMOX1 ubiquitination, thereby blocking ferroptosis and promoting the growth of ovarian cancer (OC) cells.151 The E3 ubiquitin ligase 3-hydroxy-3-methylglutaryl reductase (HRD1) exhibits decreased degradation in ovarian cancer tissues and functions as a tumor suppressor. HRD1 was found to inhibit the proliferation and colony formation of ovarian cancer cells by inducing ferroptosis through facilitation of ubiquitination-dependent SLC7A11 degradation. This finding suggests that HRD1 exerts antitumor effects by promoting ferroptosis in ovarian cancer cells by increasing SLC7A11 degradation.152
Other tumors: In bladder cancer (BCa), an important serine metabolism enzyme, phosphoglycerate dehydrogenase (PHGDH), is highly expressed. PHGDH interacts with the RNA-binding protein poly(rC)-binding Protein 2 and inhibits its ubiquitin-mediated degradation, which in turn upregulates SLC7A11 and inhibits ferroptosis, thereby promoting malignant progression.153 The PHGDH inhibitor NCT-502 enhances ferroptosis and halts tumor progression in BCa.153 This finding indicates that inhibition of PHGDH could be a therapeutic strategy for BCa. PAQR3, a member of the Progestin and AdipoQ Receptor (PAQR) family, is a newly discovered tumor suppressor, and its expression is decreased in acute lymphoblastic leukemia (ALL). PAQR3 suppresses cell proliferation and aggravates ferroptosis by increasing ubiquitin-dependent degradation of Nrf2 in ALL cells.154 Activation of autophagy was found to sensitize ALL cells to Erastin-induced ferroptosis by inhibiting ubiquitination-dependent degradation of VDAC3 mediated by the E3 ligase FBXW7,155 indicating that autophagy activation combined with ferroptosis induction is a potential therapeutic strategy for ALL. FOXM1 and Nedd4 regulate VDAC2/3 during ferroptosis in melanoma cells.156 Erastin induces FOXM1 expression to activate the transcription of Nedd4, which degrades VDAC2/3 and suppresses ferroptosis. Ablation of Nedd4 inhibits the degradation of VDAC2/3 proteins, increasing the sensitivity of cancer cells to Erastin-induced ferroptosis.156 These results suggest that Nedd4 regulates ferroptosis and highlight Nedd4 as a target for overcoming Erastin-induced resistance in melanoma cells. Silencing CDGSH iron sulfur domain 2, an iron-sulfur protein with a [2Fe-2S] cluster that is critical for cell proliferation and iron homeostasis, increases the degradation of p62 (an autophagy adaptor), leading to increased Keap1-mediated ubiquitination of Nrf2 and its subsequent degradation, thereby promoting ferroptosis in fibrosarcoma cells.157
Phosphorylation-mediated regulation of ferroptosis in cancer
Histone phosphorylation is a histone modification whose modulation is catalyzed by many protein kinases and phosphatases, such as protein phosphatase 1, mitogen- and stress-activated kinases, and Aurora B, and is achieved through the addition of phosphate groups to threonine, serine or tyrosine residues in histone tails.158 Histone phosphorylation frequently occurs early after the formation of a DNA double-strand break and mediates the recruitment of DNA damage repair proteins.159 Histone phosphorylation has been revealed to be associated with transcriptional activation. As an important epigenetic PTM, phosphorylation is strongly associated with tumorigenesis.160,161 Emerging studies suggest that phosphorylation regulates ferroptosis in cancer (Table 2 and Fig. 4).
Posttranslational modification of ferroptosis by phosphorylation and acetylation in cancer. ATF4 Activating transcription factor 4, ADCY10 adenylyl cyclase 10; β-OHB β-hydroxy-butyric acid; βTrCP beta-transducin repeat containing E3 ubiqutin protein ligase, CBP/p300 histone acetyltransferases CBP and p300, CCA cholangiocarcinoma, CRC colorectal cancer, CHAC1 glutathione specific gamma-glutamylcyclotransferase 1, CISD2 CDGSH iron sulfur domain 2, CKB creatine kinase B, DDR2 discoidin domain receptor tyrosine kinase 2, eIF2α alpha subunit of eukaryotic initiation factor 2, FSP1 ferroptosis suppressor protein 1, GC gastric cancer, GCN2 general control nonderepressible 2, GSS glutathione synthetase, HCC hepatocellular carcinoma, HMGCL ketogenesis-related hydroxy-methyl-glutaryl-CoA lyase, HSPB1 heat shock protein beta-1, HSF1 heat shock factor 1, IGF1R insulin-like growth factor 1 receptor, LUAD lung adenocarcinoma, NAT10 N-acetyltransferase 10, PKC protein kinase C, MESH1 metazoan SpoT homolog 1, RRM2 ribonucleotide reductase regulatory subunit M2, RND1 Rho family GTPase 1, TNBC, triple-negative breast cancer, YAP Yes-associated protein
Colorectal cancer: AMP-activated protein kinase (AMPK)-mediated phosphorylation of BECN1 enhances ferroptosis through binding to SLC7A11 to directly block system Xc–.162 Silencing BECN1 inhibits ferroptosis induced by the system Xc- inhibitors erastin-, sulfasalazine-, and sorafenib. Phosphorylation of BECN1 (Ser90/93/96) induced by AMPK is required for BECN1-SLC7A11 complex formation and LPO. Inhibition of PRKAA/AMPKα reduces Erastin-mediated BECN1 phosphorylation at S93/96, BECN1-SLC7A11 complex formation, and subsequent ferroptosis. Activation of the BECN1 pathway increases ferroptosis in CRC cells.162 KIF20A expression is increased in oxaliplatin-resistant CRC cell lines. Silencing KIF20A increases the sensitivity of cancer cells to oxaliplatin and suppresses NUAK1, thereby upregulating the expression of PP1β, which subsequently decreases the phosphorylation of downstream GSK3β to suppress the activation of Nrf2 and the expression of GPX4, abolishing oxaliplatin resistance in these cells.163
Hepatocellular carcinoma: AKT activated by insulin-like growth factor 1 receptor (IGF1R) signaling phosphorylates creatine kinase B (CKB) at T133, reducing its metabolic activity and increasing its binding to and phosphorylation of GPX4 at S104, which prevents HSC70 binding to GPX4, thereby abrogating degradation of GPX4 by chaperone-mediated autophagy, inhibiting ferroptosis and promoting tumor growth in mice.164 Elevated expression of ribonucleotide reductase regulatory subunit M2 (RRM2) inhibits ferroptosis in HCC cells. Ferroptotic stress induces phosphorylation of RRM2 at T33, thus promoting the RRM2-GSS interaction, which results in the translocation of RRM2 and GSS to the proteasome for simultaneous degradation.165
Gastric cancer: A recent study showed that Metazoan SpoT Homolog 1 (MESH1) is the first NADPH phosphatase regulating ferroptosis in the cytosol.166 Silencing MESH1 dramatically protects cells against ferroptosis. In GC, MESH1 knockdown upregulates the protein expression of ATF3 and ATF4, increases eIF2α phosphorylation, and induces the mRNA expression of XBPs, ATF3, and CHOP. Concurrent silencing of ATF4 restores the sensitivity of MESH1-depleted RCC4 cells to ferroptosis. Concurrent knockdown of NADK abolishes ATF3 induction.167
Breast cancer: PKCβII phosphorylates ACSL4 to boost LPO to induce ferroptosis.77 PKCβII was found to function as a critical contributor to ferroptosis by sensing initial lipid peroxides and amplifying ferroptosis-associated LPO by phosphorylating and activating ACSL4. Inhibiting the PKCβII-ACSL4 pathway attenuates ferroptosis in vitro and impedes immunotherapy-induced ferroptosis in vivo. Murine recurrent breast tumor cells are highly sensitive to ferroptosis. The receptor for collagen I, discoidin domain receptor tyrosine kinase 2 (DDR2), is upregulated in human mesenchymal breast cancer cells and ferroptosis-sensitive recurrent tumor cells. Upregulation of DDR2 increases the susceptibility of recurrent breast tumors to ferroptosis through the Hippo pathway.168 Erastin induces upregulation and phosphorylation of DDR2. Epithelial–mesenchymal transition (EMT)-driven DDR2 upregulation maintains a growth advantage but results in ferroptosis susceptibility mediated by YAP/TAZ in recurrent tumors.168 Silencing DDR2 reduces the clonogenic proliferation of recurrent tumor cells. These results reveal an important role of EMT-driven DDR2 upregulation in maintaining the growth advantage but endowing YAP/TAZ-mediated ferroptosis susceptibility in recurrent tumors, highlighting potential therapeutic strategies to eradicate recurrent breast cancer cells with mesenchymal features.168 Cystine starvation activates GCN2 to increase the phosphorylation of eIF2α and the expression of the ATF4 protein and its target gene CHAC1. Silencing CHAC1 rescues cystine depletion-induced ferroptosis in human triple-negative breast cancer (TNBC) cells.169
Non-small cell lung carcinoma: Inhibition of system XC− increases endogenous glutamate accumulation, which enhances adenylyl cyclase (ADCY)-mediated Ca2+-dependent cAMP production to stimulate protein kinase A (PKA)-associated phosphorylation and suppress glutamine-fructose-6-phosphate transaminase (GFPT1), thereby suppressing YAP expression and failing to sustain ferritinophagy-triggered transcriptional compensation of FTH1, leading to increases in the LIP and ferroptosis sensitivity.170
Osteosarcoma/prostate adenocarcinoma: Heat shock protein beta-1 (HSPB1) functions as an inhibitor of ferroptosis in cancer. Erastin enhances heat shock factor 1 (HSF1)-dependent HSPB1 expression in cancer cells. Silencing HSPB1 and HSF1 promotes but HSPB1 overexpression inhibits erastin-induced ferroptosis. Protein kinase C (PKC)-mediated HSPB1 phosphorylation inhibits ferroptosis. Inhibition of HSPB1 phosphorylation and HSF1-HSPB1 signaling boosts the anticancer activity of erastin in vivo.171
Acetylation-mediated regulation of ferroptosis in cancer
Protein acetylation is required for key cellular processes related to physiology and diseases, such as transcriptional activity, protein stability, enzyme activity, protein‒protein interactions, subcellular localization, and protein‒DNA interactions.172 Histone acetylation was first found to modulate gene transcription as early as the 1960s.173 Since the first acetylation modification of a nonhistone protein, p53, was found in the 1980s, various nonhistone proteins have been identified as targets for acetylation.172 Emerging studies suggest that acetylation regulates ferroptosis in cancer (Table 3 and Fig. 4).
Non-small cell lung carcinoma: Regulation of ferroptosis by P53 was first reported in 2015, and the associated study revealed SLC7A11 as a direct target gene of P53 for suppression.174 The acetylation-defective mutant p533KR was found to be unable to induce cell cycle arrest, senescence and apoptosis but retained the full ability to inhibit SLC7A11 expression and induce ferroptosis. Gu and colleagues revealed the role of acetylation in modulating P53-mediated ferroptosis and tumor suppression in NSCLC.175 Expression of P533KR efficiently inhibited tumor growth, which was restored by the overexpression of SLC7A11 in vivo, suggesting the important role of SLC7A11 inhibition in p53-mediated tumor suppression. However, p534KR (K98R + 3KR) lost the ability to suppress SLC7A11, thus inducing ferroptosis and tumor suppression.175 These results revealed the role of acetylation in regulating p53-mediated ferroptosis and tumor suppression. Most recent studies have shown that GINS4, a regulator of initiation and elongation during DNA replication, negatively regulates ferroptosis in lung adenocarcinoma (LUAD). Ablation of GINS4 facilitates ferroptosis.176 GINS4 suppresses P53-mediated ferroptosis by stabilizing p53 via activation of snail, which antagonizes the acetylation of P53 at K351. These results indicate that GINS4 is a potential oncogene that destabilizes p53 and then inhibits ferroptosis, thus constituting a potential therapeutic target for LUAD.176
Hepatocellular carcinoma: Dipeptidyl peptidase 4 (DPP4) is a key protein that maintains intracellular iron accumulation and LPO. Ketogenesis-related hydroxy-methyl-glutaryl-CoA lyase (HMGCL) negatively regulates cell proliferation and metastasis in HCC. HMGCL increases β-hydroxybutyric acid (β-OHB)-mediated acetylation of DPP4 at histone 3 lysine 9 (H3K9) and promotes its expression, leading to increased vulnerability of HCC cells to erastin- and sorafenib-induced ferroptosis.177 This observation suggests that HMGCL functions as a tumor suppressor by increasing ferroptosis susceptibility driven by β-OHB-mediated acetylation of DPP4.
Colorectal cancer: NAT10 negatively regulates tumorigenesis and metastasis in CRC. Upregulation of N-acetyltransferase 10 (NAT10) promotes cancer progression by inhibiting ferroptosis via N4 acetylation and stabilization of FSP1 mRNA in CRC cells.178 FSP1 mRNA undergoes N4-acetylcytidine (ac4C) modification, leading to inhibition of ferroptosis. This observation reveals that NAT10-mediated N4 acetylation of FSP1 mRNA terminates ferroptosis in colon cancer cells.
Glioma: KAT6B, a histone acetyltransferase, promotes glioma progression by inhibiting ferroptosis through epigenetic induction of STAT3.179 KAT6B expression is increased in glioma. KAT6B reverses erastin-induced ferroptosis in glioma cells, indicating that KAT6B functions as an inhibitor of ferroptosis. A mechanistic study showed that ablation of KAT6B represses the expression of STAT3. Silencing KAT6B inhibits the enrichment of RNA polymerase II (RNA pol II) and histone H3 lysine 23 acetylation (H3K23ac) on the STAT3 promoter, while loss of STAT3 reverses KAT6B-induced inhibition of ferroptosis in glioma cells.
Osteosarcoma: K139 has been identified as a novel acetylation site in human p53 accounting for p53-mediated mTOR suppression.180 The p53-4KR mutant retains the ability to inhibit mTOR activity, which is completely abolished in the p53-5KR (K136R + K98R + K117R + K161R + K162R) mutant. The 5KR mutation series in p53 abolishes its remaining tumor suppressor function. Treatment with an mTOR inhibitor was found to suppress early-onset tumor formation in P535KR/5KR mice, which was similar to that observed in p53-null mice. This finding reveals a role of p53-mediated mTOR regulation in tumor suppression.180
Methylation-mediated regulation of ferroptosis in cancer
First discovered in 1959, protein methylation is an important PTM that regulates the functions of both nonhistone and histone proteins.181,182 Histon methylation was identified in 1964.183 Currently, accumulating discoveries have revealed much of the biology of protein methylation.184 Protein methylation occurs mainly on side chains of arginine (Arg) and lysine (Lys) residues.185 The protein Arg methyltransferases (PRMTs) that use S-adenosylmethionine (SAM) as the methyl donor induce mono- or dimethylation of Arg on its side chains,185,186 whereas Lys residues may undergo mono-, di- or trimethylation (me1, me2 or me3, respectively) in a SAM-dependent manner.187 Circumstantial evidence has shown that dysregulation of protein methylation is involved in tumorigenesis.188,189 Emerging studies have suggested that methylation regulates ferroptosis in cancer (Table 4 and Fig. 5).
Epigenetic modification of ferroptosis by methylation, glcNAcylation, and SUMOylation in cancer. ALG3 Alpha 1,3-mannosyltransferase, CBS cystathionine -beta-synthase, CREB, cAMP response element-binding protein, CYP2E1 cytochrome P450 family two subfamily E member 1, EGFR epidermal growth factor receptor, ELOVL5 elongation of very long-chain fatty acid protein 5, EP300 E1A binding protein P300, FADS2 fatty acid desaturase 2, FASN fatty acid synthase, FADS1 fatty acid desaturase 1, FSP1 ferroptosis suppressor protein 1, FTH ferritin heavy chain, GALNT14 N-Acetylgalactosaminyltransferase-14, GBM glioblastoma, HMGCL ketogenesis-related hydroxy-methyl-glutaryl-CoA lyase, KDM3B histone lysine demethylase 3B, KDM4A histone lysine demethylase 4 A, β-OHB β-hydroxy-butyric acid, MM multiple myeloma, NAT10 N-acetyltransferase 10, PSAT1 phosphoserine aminotransferases 1, SDH succinate dehydrogenase, SENP1 small ubiquitin-like modifier (SUMO)-specific protease 1, TFRC transferrin receptor
Cancers of the digestive system
Increased expression of ELOVL5 and FADS1 enhances the sensitivity of mesenchymal-type GC cells to ferroptosis. ELOVL5 and FADS1 are silenced by DNA methylation in intestinal-type GCs, rendering cells resistant to ferroptosis. AA and AdA derived from linoleic acid are not generated in intestinal-type GCs. AA supplementation restores the ferroptosis sensitivity of intestinal-type GCs.190 In CRC, increased methylation levels of solute carrier family 2 member 1 (SLC2A1) greatly inhibit autophagy and ferroptosis, resulting in immunosuppression and a poor prognosis in patients.191 Aberrant methylation of the promoter inactivates PCDHB14 in HCC patients. PCDHB14 functions as a tumor suppressor to enhance cell cycle arrest, inhibit cell proliferation, and induce ferroptosis. PCDHB14 inhibits HCC progression by enhancing RNF182-mediated degradation of p65 and promotes cell sensitivity to ferroptosis in HCC by suppressing SLC7A11.129
Non-small cell lung carcinoma
There are low levels of DNA methylation in upstream regions of GPX4 and an increased level of H3K4me3. H3K27ac leads to increased expression of GPX4. Inhibiting the expression of GPX4 induces ferroptosis in cancer cells and boosts the anticancer effect of cisplatin.192 cAMP response element-binding protein (CREB) is upregulated in LUAD. Silencing CREB decreases the viability and inhibits the growth of cancer cells by promoting ferroptosis. CREB suppresses ferroptosis by binding the promoter region of GPX4, and E1A binding protein P300 (EP300) enhances this binding.193 A lysine monomethylase, SET7, directly interacts with the DUB OTUB1 to catalyze its methylation at lysine 122, inhibiting the binding of OTUB1 to the E2 ubiquitin-conjugating enzyme UBC13. SET7-mediated methylation of OTUB1 promotes ferroptosis through relieving OTUB1-mediated suppression of ferroptosis, highlighting that SET7 inhibitor treatment might enhance OTUB1 function as a therapeutic approach.194
Clear cell renal cell carcinoma
CX3CL1 overexpression attenuates the proliferation and metastasis of ccRCC cells by increasing their sensitivity to ferroptosis. The expression of CX3CL1 is associated with its DNA methylation level in ccRCC.195 Decreased expression of succinate dehydrogenase (SDH), which is responsible for oxidative phosphorylation (OXPHOS) and flux through the tricarboxylic acid (TCA) cycle, is correlated with ccRCC progression. SDH deficiency enhances tumorigenesis by inhibiting ferroptosis in ccRCC cells. High mutation rates and increased methylation in the SDH promoter lead to SDH deficiency, thereby promoting tumorigenesis through inhibition of ferroptosis.196
The promoter of the gene coding for FSP1 is hypermethylated in ALL cells, silencing the expression of FSP1 and generating selective dependency on GSH-centered antiferroptosis defenses. Expression of FSP1 in trans increases the resistance of leukemic cells to compounds targeting the GSH-dependent antiferroptosis pathway. FSP1 overexpression promotes ALL tumor growth.
Acute lymphoblastic leukemia
ALL cells are selectively sensitive to compounds that block the GSH-dependent ferroptosis defense system. The promoter of FSP1 has been found to be hypermethylated in ALL cell lines and patient biopsies. Silencing FSP1 produces selective dependency on the GSH-centered ferroptosis defense system. Overexpression of FSP1 enhances the resistance of leukemic cells to compounds that target the GSH-dependent antiferroptosis pathway, revealing metabolic vulnerability in ALL.197
Ferroptosis induction leads to changes in histone modification and DNA methylation associated with cellular senescence.
Multiple myeloma
RSL3-induced ferroptosis leads to changes in histone modification and DNA methylation related to cellular senescence.198 MM1 multiple myeloma cells are sensitive to ferroptosis induced by RSL3 and epigenetic reprogramming. Enrichment of CpG probes in genes associated with cell cycle progression and senescence was found in ferroptotic MM cells, suggesting that ferroptotic cell death is associated with an epigenomic stress response that might increase the therapeutic applicability of ferroptotic compounds.198
Fibrosarcoma
The H3K9 demethylase KDM3B functions as a potential epigenetic regulator of ferroptosis. KDM3B can inhibit erastin-triggered ferroptosis. Overexpression of KDM3B reduces H3K9 methylation and inhibits ferroptosis by upregulating SLC7A11 in cooperation with the transcription factor ATF4.199
Osteosarcoma
Upregulated KDM4A expression was found to be associated with poorer prognosis. Knockdown of KDM4A promotes ferroptosis through regulation of SLC7A11 transcription by controlling the demethylation of H3K9me3 in the SLC7A11 promoter.200
Glycosylation-mediated regulation of ferroptosis in cancer
O-GlcNAcylation, the attachment of O-linked N-acetylglucosamine (O-GlcNAc) moieties to threonine or serine residues of proteins in the nucleus, cytosol or mitochondria, is an important PTM that links nutrient flux to gene transcription in tumorigenesis.201,202 O-GlcNAcylation is dynamically and finely modulated by O-GlcNAcase (OGA) and O-GlcNAc transferase (OGT) proteins. Abnormal O-GlcNAcylation has been identified as a common characteristic in cancers due to deregulated cellular nutrient flux.203,204 Emerging studies suggest that GlcNAcylation regulates ferroptosis in cancer (Table 5 and Fig. 5).
Cancers of the digestive system: GlcNAcylated c-Jun represses ferroptosis by antagonizing the synthesis of GSH in HCC. Erastin inhibits malignant phenotypes by inhibiting the O-GlcNAcylation, protein expression, transcriptional activity and nuclear accumulation of c-Jun in HCC. An overabundance of O-GlcNAcylated c-Jun conversely inhibits ferroptosis by increasing the synthesis of GSH via increased transcription of PSAT1 and CBS.205 This observation indicates that O-GlcNAcylated c-Jun is at the core of ferroptosis and that targeting c-Jun O-GlcNAcylation might be a potential therapeutic approach for HCC.205 O-GlcNAcylation stabilizes and enhances the expression of YAP, which plays a pivotal role in controlling ferroptosis. O-GlcNAcylated YAP mediates increased ferroptosis sensitivity through a transcriptional increase in TfRC expression in HCC cells.206 Knockdown or mutation of YAP abolishes the O-GlcNAcylation-mediated increase in sensitivity to ferroptosis. The related study provided the first evidence that O-GlcNAcylation can increase the sensitivity of HCC cells to ferroptosis via YAP/TFRC, highlighting new therapeutic strategies for HCC.206 In pancreatic cancer, O-GlcNAcylation of zinc finger E-box-binding homeobox 1 (ZEB1) enhances ferroptosis in mesenchymal pancreatic cancer cells. High glucose exposure increases the O-GlcNAcylation of ZEB1, transcriptionally inducing fatty acid synthase (FASN) and fatty acid desaturase 2 (FADS2) expression, thereby resulting in ferroptosis in mesenchymal pancreatic cancer cells.207 These results indicate that glycolipid metabolism and O-GlcNAcylation play a novel role in increasing ferroptosis susceptibility in mesenchymal cancer cells, which reveals a new molecular mechanism of ferroptosis and suggests a therapeutic strategy for refractory pancreatic cancers.
Gynecologic neoplasms: Increased expression of GALNT14 is found in ovarian cancer. Silencing GALNT14 eliminates ovarian cancer cells by promoting apoptosis and ferroptosis by reducing the O-glycosylation of EGFR and promoting its degradation, thereby suppressing mTOR pathway activity.208 Combination treatment with an mTOR inhibitor and cisplatin induced apoptosis and ferroptosis, suggesting that the combination of cisplatin with an mTOR inhibitor might be a promising strategy to combat cisplatin resistance in ovarian cancer.208 Alpha 1,3-mannosyltransferase (ALG3) is involved in protein glycosylation critical for the assembly of lipid-linked oligosaccharides and in N-linked glycosylation of proteins at the luminal side of the endoplasmic reticulum (ER). Inhibition of ALG3 leads to N-linked glycosylation deficiency-mediated ferroptosis to boost the efficacy of anti-PD1 immunotherapy.209 Ablation of ALG3 was found to attenuate tumor growth in a cytotoxic T-cell-dependent manner in mice.209 Moreover, ALG3 inhibition and treatment with tunicamycin (an N-linked glycosylation inhibitor) synergize with anti-PD1 therapy to inhibit tumor growth in mouse models.209 Inhibition of ALG3 induces impairment of posttranslational N-linked glycosylation, resulting in sterol-regulated element-binding protein (SREBP1)-dependent lipogenesis and excessive lipid accumulation, which induces immunogenic ferroptosis in cancer cells and leads to the formation of a proinflammatory microenvironment, thereby boosting antitumor immune responses.
Osteosarcoma: Protein O-GlcNAcylation orchestrates both mitophagy and ferritinophagy to support ferroptosis in osteosarcoma cells.210 RSL3 modulates ferroptosis by inducing a biphasic change in protein O-GlcNAcylation. Inhibition of O-GlcNAcylation enhances ferritinophagy, leading to an increased labile iron content, thus rendering the cell more sensitive to ferroptosis. De-O-GlcNAcylation of FTH at S179 facilitates its interaction with NCOA4, resulting in labile iron accumulation to support ferroptosis.210 These results reveal links between dynamic O-GlcNAcylation and both iron metabolism and ferroptosis initiation, highlighting a potential therapeutic regimen for cancers.
SUMOylation-mediated regulation of ferroptosis in cancer
A PTM involving conjugation of small ubiquitin-like modifier (SUMO) proteins to substrate proteins, SUMOylation is involved in various cellular processes and is a vital cellular mechanism in stress responses.211 SUMOylation is also a reversible and dynamic process. SUMOylation occurs through an enzymatic cascade that consists of a dimeric SUMO-activating E1 enzyme (SAE1 and SAE2/UBA2), a single E2 enzyme (ubiquitin-conjugating enzyme 9, UBC9), and a limited set of E3 ligases.212 SUMO-specific proteases (SENPs) cooperate with SUMO molecules to modulate the SUMOylation status of a substrate protein by specifically de-SUMOylating the substrate protein. Aberrantly overactivated SUMOylation has been detected in many cancers and is involved in EMT, tumorigenesis, metastasis, drug resistance, and antitumor immunity.211,213 Emerging studies suggest that SUMOylation regulates ferroptosis in cancer (Table 6 and Fig. 5). Aberrantly increased SENP1 expression predicts a poor prognosis in patients with lung cancer. Inhibition of SENP1 inhibits the proliferation and growth of lung cancer cells. Overexpression of SENP1 inhibits Erastin- or cisplatin-induced ferroptosis. Elevated SENP1 expression mediates A20 SUMOylation to reduce the expression of ACSL4 and induce the expression of SLC7A11, thereby inhibiting ferroptosis in lung cancers.214 SENP1 functions as a ferroptosis suppressor, as determined through a novel network analysis of how A20 SUMOylation links SLC7A11 and ACSL4 in lung cancer cells. Inhibition of SENP1 enhances ferroptosis. Elevated HSP27 expression inhibits ferroptosis by inducing SUMOylation of ACSL4 to reduce its stability in GBM cells.215
N6-methyladenosine (m6A) modification-mediated regulation of ferroptosis in cancer
Accumulating studies have shown that m6A modification plays a vital role in epigenetic regulation in organisms and in the pathogenesis of malignant diseases. Aberrant m6A modification, a dynamic and reversible posttranscriptional RNA modification mediated by methyltransferases (writers), demethylases (erasers), and m6A binding proteins (readers), is associated with the development, progression, occurrence, and prognosis of cancer. Emerging studies suggest that m6A modification regulates ferroptosis in cancer (Table 7 and Fig. 6).
Epigenetic modification of ferroptosis by m6A in cancer. ALKBH5 AlkB homolog 5, ACSL3 acyl-CoA synthetase long-chain family member 3, BC breast cancer, CBS cystathionine-beta-synthase, FSP1 ferroptosis suppressor protein 1, FTH1 ferritin heavy chain 1, FTO fat mass and obesity-associated protein, GBM glioblastoma, GC gastric cancer, HCC hepatocellular carcinoma, GPX4 glutathione peroxidase 4, HDLBP high-density lipoprotein-binding protein, HPSCC hypopharyngeal squamous cell carcinoma, IGF2BP1 Insulin-like growth factor 2 mRNA binding protein 1, IGF2BP2 Insulin-like growth factor 2 mRNA binding protein 2, METTL3 methyltransferase-like protein 3, METTL14 methyltransferase -like 14, METTL16 methyltransferase-like 16, NKAP NF-κB activating protein, NPC nasopharyngeal carcinoma, NSCLC non-small cell lung carcinoma, SFPQ splicing factor proline and glutamine-rich, SLC3A2 solute carrier family 3 member 2, YTHDC2 YTH domain containing 2, YTHDF1 YTH N6-methyladenosine RNA binding protein 1, YTHDF2 YTH N6-methyladenosine RNA binding protein 2, YTHDC2 YTH domain containing 2
Non-small cell lung carcinoma: Exosomal miR-4443 promotes cisplatin resistance by enhancing METLL3-mediated m6A modification of FSP1, thereby inhibiting ferroptosis.216 Downregulation of the m6A reader YT521-B homology domain containing 2 (YTHDC2) is related to poor clinical outcomes in patients with LUAD. YTHDC2 was found to inhibit tumorigenesis in a mouse model of spontaneous LUAD. YTHDC2 inhibits cystine uptake and blocks the downstream antioxidant system. Lung tumorigenesis is rescued by supplementation with downstream cystine antioxidants in mice with pulmonary YTHDC2 overexpression. YTHDC2 inhibits LUAD tumorigenesis by suppressing the transcription of SLC7A11 mRNA and promoting its decay in an m6A-dependent manner,217 suggesting that increased cystine uptake through suppression of YTHDC2 is critical for LUAD tumorigenesis and that blocking this process may be a therapeutic approach. This observation was corroborated by other studies, which showed that YTHDC2 promoted m6A methylation and subsequently destabilized HOXA13 mRNA to suppress SLC3A2 expression, thereby inducing ferroptosis and inhibiting LUAD tumorigenesis.218 The upregulated reader protein IGF2BP3 recognizes and binds target mRNAs encoding antiferroptotic factors that can undergo METTL3-mediated m6A methylation, leading to suppression of ferroptosis and stimulation of tumorigenesis.219 The writer METTL3 promotes tumor growth and inhibits ferroptosis by stabilizing the m6A modification of SLC7A11 in LUAD.220
Hepatocellular carcinoma: Elevated expression of HDLBP, the largest RNA-binding protein, inhibits ferroptosis in HCC cells. HDLBP binds to and stabilizes lncFAL. The splicing of lncFAL is enhanced by YTHDF2 in an m6A-dependent manner. lncFAL reduces vulnerability to ferroptosis by directly binding to FSP1 and competitively abolishing Trim69-dependent polyubiquitination and degradation of FSP1.133 Upregulation of IGF2BP3 is strongly associated with early recurrence, tumor invasion, and poor prognosis in HCC. Silencing IGF2BP3 significantly promotes sorafenib-induced ferroptosis in HCC cells. Moreover, Nrf2 mRNA was identified as an important target of IGF2BP3, which stabilizes Nrf2 mRNA via m6A modification. Upregulation of IGF2BP3 inhibits sorafenib-induced ferroptosis by promoting Nrf2 mRNA stability in an m6A-dependent manner,221 suggesting a new anticancer strategy aimed at improving the efficacy of sorafenib by inhibiting IGF2BP3. METTL3-mediated m6A modification of SLC7A11 promotes ferroptosis resistance in hepatoblastoma cells. METTL3/IGF2BP1-mediated m6A modification stabilizes SLC7A11 mRNA and upregulates SLC7A11 expression by inhibiting the deadenylation process in hepatoblastoma cells.222
Thyroid cancer: ALKBH5 inhibits the progression of thyroid cancer by enhancing ferroptosis through Nrf2 inactivation mediated by decreasing the m6A level in TIAM1.223 FTO inhibits the progression of thyroid cancer by inducing ferroptosis through m6A-mediated downregulation of SLC7A11.224
Breast cancer: METTL16 promotes the progression of breast cancer by inhibiting ferroptosis by epigenetically increasing GPX4 expression via m6A modification.225 The m6A modification level is reduced due to downregulation of METTL14 in anti-HER2 therapy-resistant breast cancer. This decrease in the m6A level prevents YTHDC2-mediated degradation of FGFR4 mRNA, thus leading to the accumulation of FGFR4 in resistant breast cancer cells. FGFR4 phosphorylates GSK-3β and activates β-catenin/TCF4 signaling to increase the transcription of the SLC7A11 and FPN1 genes. Upregulation of SLC7A11 and FPN1 accelerates GSH synthesis and Fe2+ efflux, which confer anti-HER2 resistance by attenuating ferroptosis in breast cancer cells. Roblitinib, a highly selective inhibitor of FGFR4, combats anti-HER2 resistance by inducing ferroptosis in refractory HER2-positive breast cancer.226
Other tumors: In GBM, NKAP inhibits ferroptosis by recruiting SFPQ to promote SLC7A11 mRNA splicing in a manner dependent on METTL3-mediated m6A modification.227 Upregulation of FTO promotes radioresistance by repressing ferroptosis through promotion of OTUB1 expression in nasopharyngeal carcinoma (NPC).228 ALKBH5 inhibits ferroptosis by activating Nrf2 in an m6A-IGF2BP2-dependent manner in hypopharyngeal squamous cell carcinoma (HPSCC).229 HIF-1α mediates lncRNA-CBSLR expression to recruit the YTHDF2 protein and CBS mRNA to form the CBSLR/YTHDF2/CBS complex, resulting in m6A-dependent destabilization of CBS mRNA and leading to decreased methylation of the ACSL4 protein and an increase in its ubiquitin–proteasome-dependent degradation, thereby inhibiting ferroptosis in GC cells.230
Noncoding RNA-induced modulation of ferroptosis in cancer
ncRNAs, encompassing microRNAs (miRNAs), lncRNAs, and circRNAs, do not encode proteins. However, ncRNAs are considered master regulators of various cellular processes, particularly in cancers, where they are implicated in all hallmarks of cancer. Recent studies have demonstrated that ncRNAs, particularly miRNAs, lncRNAs, and circRNAs, are involved in the biological process of ferroptosis by regulating the molecular mechanism of ferroptosis in tumor cells. We refer readers to some recent excellent reviews for a detailed discussion of the roles of ncRNAs in regulating ferroptosis in cancer.231,232,233,234
Epigenetic and posttranslational modifications regulating ferroptosis in central nervous system (CNS) diseases
Accumulating evidence supports neuronal ferroptosis as a critical factor in traumatic brain injury (TBI), multiple sclerosis (MS), spinal cord injury (SCI), neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), and stroke, including spontaneous intracerebral hemorrhage (ICH), acute ischemic stroke (AIS), and subarachnoid hemorrhage (SAH); in addition, pharmacological inhibition of ferroptosis is supported as a therapeutic strategy for these diseases.19 Emerging evidence has revealed the role of epigenetic modifications and PTMs in regulating ferroptosis in CNS diseases. Below, we summarize the epigenetic modifications and PTMs regulating ferroptosis in CNS diseases (Table 8 and Fig. 7).
Epigenetic and posttranslational modification of ferroptosis in CNS disease and CVD. AIS acute ischemic stroke, BAP1 BRCA1-associated protein 1, BMSC-Exos bone marrow mesenchymal stem cells (BMSCs)-derived exosomes, CVD cardiovascular diseases, DIC doxorubicin-induced cardiomyopathy, HFD High-fat diet, Egr-1 transcription factor early growth response-1, FSP1 ferroptosis suppressor protein 1, FTH1 ferritin heavy chain 1, METTL14 methyltransferase-like 14, ME2 malic enzyme 2, MIRI myocardial ischemia/reperfusion injury, MPP+ 1-methyl-4-phenylpyridinium, MS multiple sclerosis, MSCs-exo mesenchymal stem cells-derived exosomes, NAMPT nicotinamide phosphoribosyltransferase, NHLRC1 NHL repeat-containing 1, Ptgs2 prostaglandin-endoperoxide synthase-2, TBI traumatic brain injury, SIC sepsis-induced cardiomyopathy, SPATA2 Spermatogenesis-associated protein 2, TfR1 transferrin receptor 1, TMEM43 transmembrane protein 43
Parkinson’s disease
PD is the second most common neurodegenerative disease and is a progressive condition pathologically characterized by the degeneration of nigrostriatal dopaminergic neurons located in the substantia nigra pars compacta (SNpc) of the brainstem and the presence of Lewy bodies, which consist mainly of misfolded α-synuclein, Parkin, ubiquitin, PTEN-induced kinase-1 (PINK1), and other proteins, in the surviving neurons.235,236,237,238 PD is characterized by motor and nonmotor symptoms and affects more than 2% of the population above 65 years of age.237,239 Accumulating evidence indicates that ferroptosis plays a role in the genesis of PD.19,87,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262 However, data revealing the roles of epigenetic regulation of ferroptosis by ncRNAs in PD are limited. Upregulation of the lncRNA NEAT1 was observed in 1-methyl-4-phenylpyridinium (MPP+)-treated SK-N-SH cells. Silencing lncRNA NEAT1 was found to increase cell viability through ferroptosis inhibition by sponging and suppressing miR-150-5p.41 Overexpression of miR-150-5p upregulates SLC7A11 expression by directly binding to BAP1 to suppress ferroptosis. BAP1 overexpression or miR-150-5p inhibition mitigate sh-NEAT1-mediated inhibition of ferroptosis.41 Together, these observations indicate that upregulation of NEAT1 promotes MPP+-induced ferroptosis by regulating the miR-150-5p/BAP1/SLC7A11 pathway in SK-N-SH cells.41 miR-335 was found to promote ferroptosis through the degradation of FTH1 in vivo and in vitro in 6-hydroxydopamine (6-OHDA)-induced models of PD.263 Midbrain dopamine oxidation promotes ferroptosis in dopaminergic neurons by facilitating NEDD4-mediated ubiquitination of GPX4.240
Acute ischemic stroke
Caused by arterial occlusion, AIS is the most common type of stroke, represents a significant threat to human life and is among the most frequent causes of disability and death worldwide.264,265 Emerging evidence has shown that ferroptosis plays a role in the genesis of neuronal injury after ischemic stroke.19,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292 Recently, accumulating evidence has revealed the roles of epigenetic regulation of ferroptosis via ncRNA expression, methylation, ubiquitination, and acetylation in AIS.293,294,295,296,297,298,299,300,301,302 Upregulation of the circRNA Carm1 inhibits ferroptosis through its binding to microRNA-3098-3p to downregulate ACSL4 in OGD/R-treated HT22 cells.293 Upregulation of LncRNA PVT1 promotes ferroptosis through miR-214-mediated suppression of TFR1 and P53 expression.294 miR-27a promotes ferroptosis by inhibiting Nrf2 expression during ischemic stroke.295 miR-27a aggravates cerebral ischemia/reperfusion injury (IRI) by promoting ferroptosis through inhibition of SLC7A11.296 miR-760-3p in exosomes from adipose-derived stem cells (ADSC-Exos) inhibits ferroptosis by targeting CHAC1 in neurons.297 GATA6 suppresses neuronal autophagy and ferroptosis through a miR-193b/ATG7 axis-dependent mechanism.298 LncRNA Meg3 promotes oxygen-glucose deprivation (OGD)-mediated hyperglycemic reperfusion-induced ferroptosis by upregulating p53, thereby inhibiting GPX4 expression in rat brain microvascular endothelial cells (RBMVECs).299 ELAVL1 suppresses ferroptosis-induced cerebral IRI and subsequent brain damage by inhibiting PINK1 expression through stabilization of DNMT3B mRNA.300 USP14 stabilizes NCOA4 to promote ferritinophagy-mediated ferroptosis in ischemic stroke.301 HDAC9 promotes neuronal ferroptosis through deacetylation and deubiquitination, dependently increasing HIF-1 expression and thus increasing the transcription of TfR1 and decreasing the Sp1 protein level by deacetylation and ubiquitination, leading to downregulation of GPX4 in in vitro and in vivo models of stroke.302
Intracerebral hemorrhage
ICHs are defined by brain injury resulting from acute extravasation of blood into the brain parenchyma from a ruptured cerebral blood vessel.303 Occurring spontaneously and accounting for 80% of hemorrhagic strokes and 10-15% of all strokes,303 ICH is an acute subtype of cerebral stroke. Emerging evidence has shown that ferroptosis plays a role in the genesis of secondary brain injury after ICH (SBI-ICH).19,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328 Data revealing the roles of epigenetic regulation of ferroptosis by ncRNAs in ICH are limited. LncRNA H19 protects against ICH-related injuries by regulating the microRNA-106b-5p/ACSL4 axis.329 miR-137 inhibits ferroptosis in oxyhemoglobin (oxyHb)-treated SH-SY5Y cells via the COX2/PGE2 pathway.330 Downregulation of miR-124 induces FPN expression and attenuates iron accumulation.331 Acupuncture may alleviate neuronal cell death, inflammation, and ferroptosis after ICH by downregulating miR-23a-3p.332
Traumatic brain injury
Structural and physiological disruption of brain function caused by external forces results in TBI, which is a major cause of disability and death in patients worldwide and a key injury mechanism involving the death of nerve cells.333,334,335,336,337 Emerging evidence indicates that ferroptosis plays a vital role in secondary brain injury and worsens long-term outcomes after TBI.338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353 Emerging studies have revealed the roles of epigenetic regulation of ferroptosis by ncRNAs in TBI.354 Upregulated circPtpn14 (mmu_circ_0000130) promotes ferroptosis by sponging miR-351-5p, which targets the 5-LOX mRNA for degradation, thereby upregulating the expression of 5-LOX after TBI. Decreased miR-212-5p expression has been found in TBI. Overexpression of miR-212-5p attenuates cell death by inhibiting ferroptosis through repression of Ptgs2 after TBI.354
Spinal cord injury
SCI is a severely disabling neurological condition causing primary damage to the spinal cord via compression and laceration, followed by secondary damage consisting of inflammation and ischemia, culminating in substantial loss of tissue.355,356,357,358,359 Ferroptosis plays a key role in secondary SCI, and this role is closely related to inflammation, immunity, and chronic injuries.339,340,360,361,362 Emerging studies have revealed the roles of epigenetic regulation of ferroptosis by ncRNAs and ubiquitination in SCI.354 miR-672-3p enhances functional recovery by inhibiting ferroptosis via upregulation of FSP1 in contusive SCI.363Mesenchymal stem cell (MSC) exosomal lncGm36569 attenuates neuronal dysfunction through ferroptosis inhibition by sponging miR-5627-5p to induce FSP1 upregulation.364 Silencing miR-6315 attenuates neuronal dysfunction by inhibiting ferroptosis via upregulation of GPX4.365 Downregulated USP7 expression and upregulated HMOX-1 expression were found in SCI rat models. USP7 overexpression alleviates SCI through deubiquitination of HMOX-1 and promotion of its expression, thereby reducing ferroptosis.366
Multiple sclerosis
A CNS disorder characterized by inflammation, demyelination, gliosis and neuroaxonal degeneration, MS is highly heterogeneous and affects over 2.8 million people worldwide.367,368,369,370,371 Recently, ferroptosis was shown to play a key role in the genesis of MS, and inhibition of ferroptosis was found to attenuate disease progression in an experimental autoimmune encephalitis (EAE) mouse model.372,373,374,375,376,377 Emerging studies have revealed the roles of epigenetic regulation of ferroptosis by ncRNAs and methylation in MS. The histone methyltransferase G9a promotes neurodegeneration by inducing ferroptosis by catalyzing the writing of the repressive mark H3K9me2, thereby suppressing the expression of GCLC, CBS, and GPX4.378 In addition, bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos) containing miR-367-3p were found to attenuate the severity of EAE by inhibiting ferroptosis through repression of EZH2 expression, leading to overexpression of SLC7A11.372
Epigenetic and posttranslational modifications regulating ferroptosis in cardiovascular diseases
Accumulating evidence supports ferroptosis as a critical factor in CVDs, including myocardial IRI (MIRI), doxorubicin (DOX)-induced cardiomyopathy (DIC), cardiac fibrosis (CF), heart failure, high-fat diet (HFD)-induced cardiac injury, cardiac hypertrophy, aortic dissection (AD), and septic cardiomyopathy (SCM). Below, we summarize the epigenetic regulation of ferroptosis in CVDs (Table 9 and Fig. 7).
Myocardial ischemia/reperfusion injury
Myocardial reperfusion strategies and reoxygenation are the preferred and effective treatments for acute myocardial infarction (AMI).379,380 However, reperfusion inevitably causes cardiomyocyte death, increases the infarct size, and aggravates the condition; these events are collectively referred to as MIRI.381 MIRI leads to energy metabolism disruption and oxidative stress, among other issues.382 A recent novel study revealed a role of ferroptosis in MIRI in murine models, establishing a correlation between cardiac cell death and ferroptosis in vivo.383,384 Thereafter, emerging studies have attempted to elucidate the pathophysiological role of ferroptosis in the genesis of MIRI.92,382,383,385,386,387,388,389,390,391,392,393,394,395 There are also emerging studies that have revealed the roles of epigenetic regulation of ferroptosis by ncRNAs, ubiquitination, and phosphorylation in MIRI385,396,397,398,399,400,401,402,403,404,405 (Table 9). CircRNA FEACR alleviates MIRI through inhibition of ferroptosis by interacting with nicotinamide phosphoribosyltransferase (NAMPT), which upregulates NAMPT-dependent sirtuin1 (Sirt1) expression, thereby reducing the forkhead box protein O1 (FOXO1) acetylation level to promote its transcriptional activity, eventually upregulating the transcription of FTH1.396 Upregulation of LncRNA SEMA5A-IT1 protects cardiomyocytes against hypoxia/reoxygenation (H/R) injury partially through inhibition of ferroptosis by sponging miR-143-3p to upregulate SLC7A11.397 miR-210-3p attenuates myocardial cell injury induced by H/R through inhibition of ferroptosis by downregulating TfR1.398 miR-190a-5p promotes ferroptosis through inhibition of GLS2 expression.399 MiR-199a-5p promotes cardiomyocyte death in OGD/reperfusion (OGD/R)-treated H9c2 cells by inducing ferroptosis via downregulation of GPX4 through inhibition of the Akt/eNOS signaling pathway.400 Upregulated expression of miR-214-3p promotes MIRI by inducing ferroptosis through suppression of ME2.401 Egr-1-mediated upregulation of miR-15a-5p promotes MIRI by inducing ferroptosis through suppression of GPX4.402 Upregulated expression of frataxin alleviates cardiomyocyte ferroptosis. NHL repeat-containing 1 (NHLRC1) is an E3 ligase that mediates ubiquitin-mediated degradation of frataxin.403 USP7 promotes MIRI by inducing ferroptosis via activation of the p53/TfR1 pathway.385 USP22 alleviates MIRI by inhibiting ferroptosis via the SIRT1-p53/SLC7A11 axis.404 The expression of SIRT1, USP22, and SLC7A11 is inhibited by IRI, whereas increased expression of p53 is found in affected myocardial tissues. Conversely, overexpression of USP22, SIRT1, or SLC7A11 inhibits IRI and increases the viability of cardiomyocytes by inhibiting ferroptosis.404 Upregulated expression of HIP-55 alleviates cardiomyocyte ferroptosis and MI injury.405 HIP-55 is phosphorylated by AKT at S269/T291, leading to negatively regulation of MAP4K1 by AKT to attenuate MI injury in a site-specific manner, as evidenced by the finding that S269A/T291A-mutated HIP-55 (HIP-55AA) decreased the interaction between HIP-55 and MAP4K1, resulting in loss of ferroptosis inhibition.405
Doxorubicin-induced cardiomyopathy
Anthracycline-based chemotherapy can lead to the progressive development of cardiomyopathy. Although DOX is an effective chemotherapeutic agent prescribed to treat breast, ovarian, and gastrointestinal cancers, it causes cardiotoxicity, resulting in DCM, which leads to congestive heart failure.25,406 The exact mechanisms of DOX-induced cardiotoxicity remain poorly understood. However, emerging studies have attempted to elucidate the pathophysiological role of ferroptosis in the genesis of DCM.25,383,407,408,409 In addition, emerging studies have revealed the roles of epigenetic regulation of ferroptosis by m6A modification, other methylation modifications, phosphorylation, and ubiquitination in DCM410,411,412,413,414,415 (Table 9). METTL14 promotes ferroptosis in DOX-induced cardiomyocytes by mediating IGF2BP1 m6A-dependent stabilization of KCNQ1OT1 to sponge miR-7-5p, thereby increasing the level of the transferrin receptor.410 Upregulated expression of PRMT4 aggravates DIC by promoting ferroptosis via an interaction with Nrf2 to promote its enzymatic methylation, thereby suppressing GPX4 expression.411 Activation of AMPK α2 attenuates DOX-induced cardiotoxicity by inhibiting ferroptosis.412 TRIM21 ablation protects against DIC through inhibition of ferroptosis by enhancing sequestration of Keap1 by P62.413 MITOL knockdown reduced susceptibility to DOX in cultured cardiomyocytes stressed with DOX.414 Overexpressed SPATA2 recruits CYLD to promote ferritinophagy by decreasing NCOA4 ubiquitination and ferritin expression and promote ferroptosis by increasing ACSL4 expression.415
Cardiac fibrosis
Knockout of SIRT3 results in increased p53 acetylation and ferroptosis through downregulation of GPX4 in mouse hearts. p53 acetylation-induced ferroptosis is partially involved in SIRT3-mediated CF in myofibroblasts.416 MiR-375-3p promotes CF by accelerating ferroptosis in cardiomyocytes by downregulating GPX4.417
Heart failure
circSnx12 functions as an endogenous sponge to bind miR-224-5p, and FTH1 has miRNA binding sites in its 3’UTR.418 Downregulated expression of PGAM5 promotes ferroptosis in models of heart failure by decreasing Keap1 ubiquitination, thereby reducing the activation of Nrf2.419
HFD-induced cardiac injury
The IL-6/STAT3 axis exacerbates cardiac injury by increasing NCOA4-mediated ferritinophagy and ferroptosis in HFD-fed mice.420
Cardiac hypertrophy
CD147 promotes cardiac remodeling and dysfunction by promoting ferroptosis in a glycosylation-dependent manner by binding to TRAF2 to activate the downstream TRAF2-TAK1 axis.421
Aortic dissection
Upregulated expression of METTL3 facilitates ferroptosis in human aortic smooth muscle cells by promoting the degradation of SLC7A11 and FSP1 mRNA.422
Septic cardiomyopathy
Seventy percent of patients with sepsis have SCM, which is the leading cause of sepsis-related mortality and morbidity.423,424 Ferroptosis was recently found to play a key role in the genesis of SCM, and inhibition of ferroptosis attenuates SCM progression.425,426,427,428,429 Thus, ferroptosis is involved in SCM. Emerging studies have revealed the roles of epigenetic regulation of ferroptosis in SCM410,411,412,413,414,415 (Table 9). TMEM43 knockdown promotes lipopolysaccharide (LPS)-induced cardiac injury and dysfunction in mice by inducing ferroptosis by increasing the levels of P53 and ferritin and decreasing the levels of GPX4 and SLC7A11.425
Epigenetic and posttranslational modifications regulating ferroptosis in liver diseases
Accumulating studies have shown that ferroptosis plays a role in the genesis of liver diseases,29,30 and pharmacological induction and inhibition of ferroptosis show significant potential utility for the treatment of hepatic disorders.430,431,432,433,434 Accumulating evidence indicates epigenetic modifications regulate ferroptosis in liver diseases, including acute liver injury (ALI), nonalcoholic fatty liver disease (NAFLD), liver fibrosis, hepatic ischemia/reperfusion injury, and toxin-mediated hepatic toxicity. In this section, we summarize the epigenetic regulation of ferroptosis by ubiquitination, phosphorylation, and acetylation in liver diseases (Table 10 and Fig. 8).
Epigenetic and posttranslational modification of ferroptosis in Liver diseases and Lung diseases. AUF1 AU-rich element (ARE)-binding factor 1, BRD7 bromodomain-containing protein 7, COPD chronic obstructive pulmonary disease, GCN5L1 mitochondrial general control of amino acid synthesis 5 like 1, IRI ischemia/reperfusion injury, LOXs lipoxygenases, NETs neutrophil extracellular traps, Ptgs2 prostaglandin-endoperoxide synthase 2, STAT3 signal transducer and activator of transcription 3, TfR1 transferrin receptor 1, TMEM16A transmembrane member 16A, TMEM16A transmembrane member 16A
Acute liver injury
ALI is the primary cause of liver diseases and is associated with high morbidity and mortality, causing 3.5% of deaths worldwide.435 Various hepatotoxic factors, including lipid deposition, viruses, and drugs, can induce ALI. Emerging studies have suggested that ferroptosis plays a role in the genesis of ALI436,437,438,439,440,441,442,443,444 and have revealed the roles of epigenetic regulation of ferroptosis by ubiquitination in ALI410,411,412,413,414,415 (Table 10). Mesenchymal stem cells (MSCs) and MSC-derived exosomes (MSC-Exos) alleviate injury through ferroptosis inhibition by decreasing the mRNA levels of Ptgs2 and LOXs while increasing the protein level of SLC7A11 in CCl4-induced ALI. MSC-Exo-induced expression of the SLC7A11 protein was found to be accompanied by increases in CD44 and OTUB1 expression. OTUB1-mediated deubiquitination rescued CCl4-triggered ubiquitination of SLC7A11, thus stabilizing SLC7A11 to activate system XC− and thereby preventing hepatocyte ferroptosis.445 The overexpressed ubiquitin E3 ligase HUWE1 inhibits ferroptosis by targeting TfR1 for ubiquitination and proteasomal degradation in the context of CCl4-induced liver injury, suggesting that HUWE1 functions as an inhibitor to mitigate ALI by antagonizing both aberrant iron accumulation and ferroptosis.446
Liver fibrosis
Liver fibrosis is a pathological state and abnormal repair response to chronic liver injury that is characterized by diffuse and progressive excessive deposition of extracellular matrix (ECM) in the liver. Liver fibrosis typically results from chronic liver damage resulting from infection, toxin or drug exposure, NAFLD, cholestasis, or autoimmune insults.447,448 Liver fibrosis has become one of the leading causes of liver disease globally and often progresses to liver cirrhosis and HCC. Accumulating evidence has shown that ferroptosis is involved in the pathogenesis of liver fibrosis.449,450,451,452,453,454,455,456,457 Recent studies have also shown the roles of epigenetic regulation of ferroptosis by phosphorylation458 and ubiquitination459,460,461 in liver fibrosis. Upregulated expression of ELAVL1 induces liver fibrosis by promoting ferritinophagy in hepatic stellate cells (HSCs).459 Downregulation of ZFP36 mediated by the ubiquitin ligase FBXW7/CDC4 promotes activation of ferritinophagy and induction of ferroptosis in human HSCs.458 BRD7 upregulation induced by xCT inhibition, GPX4 inhibition, and GSH depletion triggers mitochondrial translocation of p53 via direct binding to the N-terminal transactivation domain, thus increasing mitochondrial iron and LPO and eventually causing ferroptosis.460 TRIM26 mediates the ubiquitination of SLC7A11 to promote ferroptosis in HSCs, thereby suppressing liver fibrosis.461
Nonalcoholic fatty liver disease
GCN5L1 expression has been found to be increased in human patients and mice with nonalcoholic steatohepatitis (NASH). GCN5L1 acetylates CypD to enhance its binding to ATP5B, resulting in the release of mitochondrial ROS (mtROS) into the cytoplasm, which promotes ferroptosis in hepatocytes and induces the accumulation of high mobility group box 1 in the microenvironment, thereby inducing the formation of neutrophil extracellular traps (NETs).462
Hepatic ischemia/reperfusion injury
TMEM16A ubiquitinates and degrades GPX4 to induce ferroptosis. TMEM16A deficiency alleviates hepatic IRI by suppressing GPX4-mediated ferroptosis.463
Toxin-mediated hepatic toxicity
Ethyl carbamate induces ferroptosis by inhibiting the activation of Nrf2 through suppression of its phosphorylation and nuclear translocation, thereby inhibiting SLC7A11 expression and leading to GSH depletion.464 Glyphosate triggers ferroptosis in hepatocytes by suppressing Nrf2 via blockade of Nrf2 phosphorylation and nuclear translocation, resulting in GSH depletion and inhibition of GPX4.465
Epigenetic and posttranslational modifications regulating ferroptosis in lung diseases
Accumulating studies have shown epigenetic regulation of ferroptosis in the genesis of lung diseases, including chronic obstructive pulmonary disease (COPD), acute lung injury, pulmonary fibrosis, and sepsis-induced acute lung injury. In this section, we summarize the epigenetic regulation of ferroptosis in these lung diseases (Table 11 and Fig. 8).
Acute lung injury
A common and critical illness caused by both pulmonary and extrapulmonary factors, acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS), result in high morbidity and mortality and have no effective treatments.466,467 Accumulating evidence has revealed that ferroptosis is involved in the pathogenesis of ALI.42,468 Recent studies have also shown the roles of epigenetic regulation of ferroptosis by phosphorylation469,470 and acetylation471,472 in acute lung injury. Signal transducer and activator of transcription 6 (STAT6) alleviates acute lung injury through ferroptosis inhibition by competitively binding the critical acetyltransferase for p53 acetylation, i.e., CREB-binding protein, to suppress p53 acetylation and transcriptionally restore the expression of SLC7A11.471 Decreased SIRT1 expression triggers heat stress-induced lung epithelial cell injury through ferroptosis induction by increasing the acetylation of P53, which transcriptionally inhibits SLC7A11.472 mtROS-initiated dysfunction of mitochondria-associated endoplasmic reticulum membranes (MAMs) is partially implicated in arsenic-induced ferroptosis and ALI.469 Nrf2 was found to activate and cooperate with STAT3 to upregulate SLC7A11 to inhibit ferroptosis in an intestinal ischemia/reperfusion-induced acute lung injury (IIR-ALI) model.470
Sepsis-associated acute lung injury (SALI)
Accumulating evidence has demonstrated that ferroptosis is involved in the pathogenesis of SALI.43,473,474,475,476,477,478,479 Recent studies have also shown the roles of epigenetic regulation of ferroptosis by phosphorylation and acetylation in SALI. NETs induce ferroptosis through METTL3-induced m6A modification of GPX4 in the pathogenesis of sepsis-associated ALI.473 miR-125b-5p in ADSC-Exos alleviates sepsis-induced ferroptosis in mouse pulmonary microvascular endothelial cells (MPVECs) by upregulating Keap1/Nrf2/GPX4 expression, hence ameliorating sepsis-induced acute lung injury.480 Inhibition of MUC1 aggravates lung injury by triggering ferroptosis via a reduction in the phosphorylation level of GSK3β and an increase in Keap1 expression, thereby inhibiting the activation of Nrf2 to decrease GPX4 expression.474 FBXW7 mediates the protein degradation of AU-rich element (ARE)-binding factor 1 (AUF1). AUF1 alleviates sepsis-induced acute lung injury through ferroptosis inhibition by upregulating Nrf2 and downregulating ATF3.475 ncRNAs are also involved in the modulation of SALI by regulating ferroptosis. Septic conditions were found to upregulate the expression of circEXOC5 in both in vivo and in vitro sepsis models. CircEXOC5 exacerbates SALI through ferroptosis induction by stabilizing ACSL4 mRNA via PTBP1 binding.479 Silencing circEXOC5 inhibits lung injury by alleviating ferroptosis, as evidenced by upregulated GPX4 protein expression, decreased ROS levels, and decreased ACSL4 expression.479
Chronic obstructive pulmonary disease
NF-κB/RelA-mediated PRMT7 upregulation induces monomethylation of histones at enhancers and can regulate Rap1a expression, which is crucial for MAPK signaling downstream of G-protein coupled receptor activation, integrin activation, and the consequent adhesion and migration abilities of monocytes. ALOX5-mediated release of LTB4 in inflammatory macrophages upregulates ACSL4 in AT2 cells, thereby promoting tissue injury through increasing susceptibility to cigarette smoke-induced ferroptosis.481 Nrf2 promoter hypermethylation-induced inhibition of Nrf2 expression induces ferroptosis by inhibiting GPX4 in human bronchial epithelial (HBE) cells challenged with cigarette smoke extract.482 Cigarette smoke-induced USP14 downregulation aggravates bronchial epithelial cell ferroptosis through proteasomal degradation of MFG-E8.483
Epigenetic and posttranslational modifications regulating ferroptosis in kidney diseases
Accumulating studies have shown that ferroptosis plays a role in the genesis of kidney diseases.35 Accumulating evidence has shown epigenetic regulation of ferroptosis in kidney diseases, including acute kidney injury (AKI),484,485 diabetic nephropathy (DN),486 renal fibrosis,487 renal IRI (RIRI),488,489,490,491,492 sepsis-induced acute kidney injury (SAKI),493 toxin-mediated kidney toxicity,494 and crystal nephropathies.495 Below, we summarize the epigenetic regulation of ferroptosis by phosphorylation, ncRNAs, deacetylation, and ubiquitination in kidney diseases (Table 12 and Fig. 9).
Epigenetic and posttranslational modification of ferroptosis in Kidney disease. AKI Acute kidney injury, ALK activin receptor-like kinase, CHAC1 Cation transport regulator-like protein 1, DNMT1 DNA methyltransferase 1, FMR1 FMRP translational regulator 1, IRI ischemia/reperfusion injury, SAKI sepsis-induced acute kidney injury, TBK1 TANK-binding kinase 1, TIGAR TP53-induced glycolysis and apoptosis regulator
Acute kidney injury
AKI is caused by sudden loss of excretory kidney function, leading to increased serum creatinine, decreased urine output, or both.496,497 The various etiologies of AKI include kidney ischemia, exposure to nephrotoxins, dehydration and sepsis.498 AKI develops in ~10–15% of inpatients. Kidney damage or dysfunction can occur over a longer period or follow AKI along a continuum with acute and chronic kidney disease.496 Ferroptosis is closely associated with the development of AKI.44,45,499,500,501 Accumulating evidence has revealed epigenetic regulation of ferroptosis by phosphorylation and ncRNAs in AKI. Translocation of P66Shc to mitochondria promotes cisplatin-induced AKI by promoting ferroptosis.484 MicroRNA-214-3p aggravates cisplatin-induced AKI through ferroptosis induction by targeting GPX4.485
Diabetic nephropathy
DN, also called diabetic kidney disease (DKD), results from microvascular damage sustained as a result of diabetes, leading to proximal tubule injury.502 DKD is the leading cause of kidney failure worldwide, affecting approximately half of patients with type 2 diabetes and one-third of patients with type 1 diabetes. The characteristics of DKD are the accumulation of ECM, hypertrophy and fibrosis in kidney glomerular and tubular cells.503
Increasing evidence has shown that ferroptosis plays a vital role in the genesis of DKD.504,505,506,507,508,509 A recent study also showed the role of epigenetic regulation of ferroptosis by ncRNAs in DKD. Downregulated expression of mmu_circRNA_0000309 results in a decrease in its competitive sponging of miR-188-3p, subsequently promoting GPX4 expression, thereby blocking ferroptosis-dependent mitochondrial damage and podocyte apoptosis. In addition, overexpression of GPX4 counteracts mmu_circRNA_0000309 silencing-mediated ferroptosis in germacrone-exposed MPC5 cells.486
Renal fibrosis
Renal fibrosis is a common final outcome of a wide variety of chronic kidney disease (CKD) and is characterized by excessive deposition of ECM that leads to tissue scarring.510 Evidence has shown that ferroptosis is involved in the pathogenesis of kidney fibrosis.432,487,511,512,513,514,515,516 A recent study also showed the role of epigenetic regulation of ferroptosis by deacetylation in kidney fibrosis. Sirtuin 1-mediated p53 deacetylation was found to attenuate calcium oxalate deposition-induced renal fibrosis by suppressing ferroptosis.487
Renal ischemia/reperfusion injury
RIRI is the main cause of AKI and contributes to rapid renal dysfunction, morbidity and mortality in patients with a wide range of injuries.517 Evidence has demonstrated that ferroptosis is involved in the pathogenesis of RIRI.518,519,520,521,522,523 Increasing evidence shows the roles of epigenetic regulation of ferroptosis by ncRNAs and ubiquitination in RIRI. Upregulated expression of miR-20a-5p attenuates IRI and postischemic renal fibrosis through ferroptosis inhibition by repressing ACSL4.488 Upregulated expression of miR-182-5p and miR-378a-3p leads to ferroptosis in the context of renal injury by downregulating GPX4 and SLC7A11, respectively, in the rat kidneys subjected to ischemia/reperfusion.489 Increased USP14 expression has been found in H/R-exposed HK-2 cells and in kidney tissues of mice subjected to I/R. Inhibiting USP14 suppresses H/R-induced ferroptosis in HK-2 cells.490 TRIM21 aggravates ischemia/reperfusion-induced AKI by promoting ferroptosis via ubiquitination of GPX4.491 Inhibiting USP7 attenuates RIRI through ferroptosis inhibition by decreasing the ubiquitination of TBK1 and promoting DNMT1-mediated methylation of FMR1.492
Sepsis-induced acute kidney injury
SAKI is a common critical illness characterized by rapid sepsis-associated deterioration of renal function; it is one of the leading causes of death worldwide and is a common and life-threatening complication in hospitalized and critically ill patients.524,525,526,527 SAKI leads to a greatly increased risk of CKD, cardiovascular events and death. Novel findings suggest that ferroptosis plays a pathological role in SAKI.528,529,530 Recent evidence has shown the role of epigenetic regulation of ferroptosis by ncRNAs in SAKI, demonstrating that LPS increases the phosphatidylcholine content and the activity of LPCAT3 and decreases the expression of miR-124-3p.1 in human renal tubular epithelial cells in a SAKI model.531 miR-124-3p.1 overexpression in turn suppresses cell injury by inhibiting LPCAT3-related ferroptosis, suggesting that miR-124-3p.1 is a ferroptosis inhibitor.531
Modulation of epigenetic and posttranslational modifications regulating ferroptosis for disease therapy
Modulation of epigenetic modifications and PTMs regulating ferroptosis for cancer therapy
Epigenetic modifications modulate multiple aspects of cancer biology, and during the last decade, this observation has increased the interest in developing epigenetic strategies to impact tumor growth and drug resistance, thereby combating cancers.121,122,532 Unlike genetic mutations, dysregulated epigenetic mechanisms and PTMs can feasibly be targeted by small molecule compounds to treat cancers (Table 13).
Hepatocellular carcinoma
Tiliroside, a potent TBK1 inhibitor, increases the sensitivity of HCC to sorafenib by targeting TBK1 to induce ferroptosis by decreasing the phosphorylation of p62 and the affinity of p62 for Keap1 and promoting Keap1-mediated Nrf2 ubiquitination and degradation in HCC.533 Corosolic acid can increase the sensitivity of HCC to ferroptosis by inhibiting GSH synthesis via HERPUD1, which decreases MDM2 ubiquitination and promotes the ubiquitination of GSS, thereby inhibiting GSH synthesis to enhance ferroptosis in HCC.534 DHA triggers ferroptosis by enhancing the formation of the PEBP1/15-LO complex and promoting cell membrane LPO. DHA promotes PEBP1 protein expression through inhibition of its ubiquitination and degradation.535 Polyphyllin VI induces ferroptosis by inhibiting STAT3 phosphorylation, which inhibits GPX4 expression in HCC.536 Anisomycin activates p38MAPK to induce ferritinophagy through the phosphorylation of histone H3 on serine 10 (p-H3S10) in HCC.537
Non-small cell lung carcinoma
Bufotalin induces ferroptosis by facilitating the ubiquitination and degradation of GPX4 in NSCLC.538 Sanguinarine triggers ferroptosis by destabilizing GPX4 by promoting its ubiquitination and degradation mediated by its E3 ligase STUB1 in NSCLC.539 Selenite induces ferroptosis by activating the p38-ATF4-DDIT3 axis. Selenite also alters the cellular DNA methylation machinery by upregulating TET1 and downregulating DNMT1. Low-dose selenite treatment enhances the antitumor activity of osimertinib in EGFR-mutant H1975 NSCLC cells and of adagrasib in KRAS-mutant H358 NSCLC cells.540
Breast cancer
Eupaformosanin significantly decreases the viability of TNBC cells by inducing ferroptosis via ubiquitination of mutant p53.541 DMOCPTL induces ferroptosis through ubiquitination of GPX4 in breast cancer cells.542 NN3 kills p53-positive breast cancer cells by promoting ferroptosis through downregulation of the SLC7A11 pathway by triggering the ubiquitination and proteasome-dependent degradation of PARP1.543 Ketamine induces ferroptosis through GPX4 repression by decreasing the occupancy of KAT5 in its promoter region, thus inhibiting the enrichment of H3K27ac and RNA pol II, in breast cancer cells.544
Glioblastoma/glioma
ALZ003 induces FBXL2-mediated AR ubiquitination and degradation. ALZ003 significantly inhibits glioblastoma cell survival by inducing ferroptosis.545 Paeoniflorin inhibits glioma growth by inducing ferroptosis through upregulation of NEDD4L to mediate STAT3 ubiquitination-mediated repression of Nrf2 and GPX4 in glioma.546 Myrislignan induces ferroptosis through the Slug-SLC7A11 axis by inactivating NF-κB signaling through blockade of p65 phosphorylation in GBM cells.547
Pancreatic cancer
QD394 induces ferroptosis by inhibiting STAT3 phosphorylation, thereby inhibiting GPX4 expression in pancreatic cancer.548
Esophageal squamous cell carcinoma (ESCC)
Allicin may induce ferritinophagy by increasing AMPK phosphorylation and decreasing mTOR in ESCC.549
Ovarian cancer
Eriodictyol induces ferroptosis by downregulating Nrf2 phosphorylation, thereby decreasing the protein levels of SLC7A11 and GPX4 in ovarian cancer.550
Thyroid cancer
RSL3 activates ferroptosis by suppressing mTOR-triggered autophagy. Inhibiting GPX4 mirrored the effect of RSL3 on mTOR pathway suppression in thyroid cancer.551
Modulation of epigenetic and posttranslational modifications regulating ferroptosis for CNS disease therapy
A therapeutic regimen for pharmacologically inhibiting ferroptosis to treat disease has been convincingly established in CNS diseases, especially in neurodegenerative diseases and stroke.19 Small molecule compounds also feasibly target dysregulated epigenetic mechanisms and PTMs in CNS diseases (Table 14).
Intracerebral hemorrhage
Paeonol, a naturally occurring phenolic agent extracted from Cortex Moutan, exerts neuroprotective effects in patients with many CNS diseases.552,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573 Paeonol inhibits ferroptosis in neurons in ICH by downregulating HOTAIR-dependent UPF1-mediated degradation of ACSL4.312 Isorhynchophylline (IRN), a tetracyclic oxindole alkaloid extracted from Uncaria rhynchophylla, has diverse biological activities, such as antioxidant, neuroprotective and anti-inflammatory activities.574,575,576,577,578,579,580,581,582,583 Isorhynchophylline attenuates cell damage by inhibiting ferroptosis via upregulation of miR-122-5p and SLC7A11 mRNA expression and inhibiting p53 expression in ferric ammonium citrate (FAC)-treated hippocampal HT-22 cells. The protective effects of IRN against FAC-induced ferroptosis were weakened by miR-122-5p knockdown.313
Parkinson’s disease
(-)Clausenamide (Clau), a scavenger of lipid peroxidation products, is an alkaloid that is isolated from the plant Clausena lansium (Lour.) and exhibits neuroprotective activities both in vivo and in vitro.574,575,576,577,578,579,580,581,582,583 Clau prevents the nuclear translocation of ALOX5 by directly interacting with Ser663 of ALOX5, the PKCα phosphorylation site.240
Traumatic brain injury
Melatonin, an indole hormone that contributes to neuroprotection, exerts a neuroprotective effect by inhibiting ferroptosis.584,585,586,587 A recent study showed that melatonin ameliorates neurological deficits by inhibiting neuroinflammation and ferroptosis via MT2/IL-33/ferritin H signaling in TBI.588 Melatonin improves brain function in mice after TBI by inhibiting ferroptosis and ER stress by reducing the expression of circPtpn14 (mmu_circ_0000130), which sponges miR-351-5p to downregulate 5-LOX.589
Multiple sclerosis
The G9a inhibitor UNC0642 reduces inflammation-induced neuronal loss and improves clinical outcomes through restoring the expression of antiferroptotic genes. The neuronal expression of antiferroptotic genes was found to be decreased in MS brain tissue but was increased by G9a inhibition in human neuronal cultures.378
Modulation of epigenetic modifications regulating ferroptosis for CVD therapy
Increasing evidence has suggested that ferroptosis may represent a therapeutic approach for CVD. Some small molecule compounds can target dysregulated epigenetic mechanisms in liver diseases (Table 14).
Aortic dissection
BRD4770 attenuates aortic dilation by inhibiting the inflammatory response and ferroptosis.590 Liproxstatin-1 inhibits BAPN-induced aortic aneurysm and dissection (AAD) in mice. Inhibition of METTL3 expression or ferroptosis is an effective intervention strategy for AD.422 Ferrostatin-1 (Fer-1) abolishes the detrimental role of PRMT4-mediated ferroptosis in DIC.411
Disseminated intravascular coagulation (DIC)
Metformin (MET) improves cardiac function by inhibiting ferroptosis through activation of AMPKα2 phosphorylation.412 Ferrostatin-1 suppresses the exacerbation of DOX-induced myocardial damage in MITOL-knockout hearts.414
Cardiac fibrosis
C646 attenuates ferroptosis by inhibiting p53 acetylation in H9c2 myofibroblasts.416 Treatment of SIRT3-cKO mice with ferrostatin-1 was found to lead to significant reductions in ferroptosis and CF.416 Ferrostatin-1 increases the antioxidant capacity of cardiac fibroblasts, reduces GPX4-mediated ferroptosis and alleviates I/R-induced CF.417
HFD-induced cardiac injury
Celastrol induces ferroptosis resistance via AKT/GSK3β signaling in HFD-induced cardiac injury.591 Piperlongumine inhibits ferritinophagy-mediated ferroptosis in cardiomyocytes by reducing the phosphorylated STAT3 level.420
Myocardial ischemia/reperfusion injury
Inhibition of miR-190a-5p upregulates GLS2, resulting in decreased ferroptosis, and this effect could be blocked by the GLS2 inhibitor compound 968.399
Modulation of epigenetic modification and posttranslational modifications regulating ferroptosis for liver disease therapy
Emerging evidence has suggested that ferroptosis may represent a novel therapeutic approach for liver diseases. Dysregulated epigenetic mechanisms and PTMs can also be feasibly targeted by small molecule compounds in liver diseases (Table 14).
Liver fibrosis
Berberine attenuates liver fibrosis in mice by inducing ROS-mediated HSC ferroptosis.592 Recombinant FGF21 inhibits liver fibrosis by inhibiting hepatocyte ferroptosis through promotion of HO-1 ubiquitination and degradation and Nrf2 activation.593 Artemether alleviates liver fibrosis in vivo and in vitro by inducing HSC ferroptosis through inhibition of IRP2 ubiquitination, thereby inducing an increase in the iron content in HSCs.594
Nonalcoholic fatty liver disease
Urolithin C ameliorates NAFLD by regulating the AMPK-ferroptosis axis, maintaining the intestinal mucosal barrier and counteracting gut dysbiosis.595
Toxin-mediated hepatic toxicity
Ferrostatin-1 and tert-butylhydroquinone (tBHQ) reverse ethyl carbamate-induced liver dysfunction and inflammation by inhibiting ferroptosis in BALB/c mice.464 Ferrostatin-1 and tBHQ were also found to attenuate liver damage resulting from glyphosate-triggered ferroptosis in a mouse model.465
Acute liver failure
AGK2 alleviates thioacetamide-induced acute liver failure by inhibiting ferroptosis by regulating the MFN2-PERK pathway.596
Modulation of epigenetic and posttranslational modifications regulating ferroptosis for lung disease therapy
Emerging evidence has suggested that targeting ferroptosis may represent a novel therapeutic approach for lung diseases. Dysregulated epigenetic mechanisms and PTMs can also be feasibly targeted by small molecule compounds in lung diseases (Table 14).
Acute lung injury
MitoQ pretreatment counteracts arsenic-induced pulmonary ferroptosis and ALI.469 Ferrostatin-1 mitigates arsenic-triggered mitochondrial damage and ferroptosis in MLE-12 cells.469 PERK inhibitors mitigate arsenic-induced ferroptosis in MLE-12 cells.469 Ferrostatin-1 alleviates lung injury and pulmonary edema.470
Sepsis-induced acute lung injury
Obacunone alleviates LPS-induced acute lung injury by inhibiting ferroptosis through inhibition of the ubiquitin-mediated proteasomal degradation of Nrf2, thereby upregulating GPX4 and SLC7A11.597
Pulmonary epithelial senescence
SIRT3 activation by melatonin alleviates PM2.5-induced senescence and ferroptosis in mice by deacetylating p53 at lysine 320 (K320).598
Chronic obstructive pulmonary disease
hMFG-E8 ameliorates ferroptosis induced by cigarette smoke extract in BEAS-2B cells and HBE cells.483
Modulation of epigenetic and posttranslational modifications regulating ferroptosis for kidney disease therapy
An increasing body of evidence indicates that targeting ferroptosis may represent a therapeutic approach for kidney disease. Dysregulated epigenetic mechanisms and PTMs can also be feasibly targeted by small molecule compounds in kidney diseases (Table 14).
Diabetic nephropathy
Germacrone ameliorates DN by inhibiting ferroptosis through upregulation of mmu_circRNA_0000309, which sponges miR-188-3p, subsequently upregulating GPX4 expression.486
Renal IRI
The small molecule USP14 inhibitors IU1 and NAC ameliorate renal injury in I/R mice.490 The JAK2 inhibitor fedratinib downregulates TRIM21 expression and reduces damage both in vivo and in vitro, and these effects are correlated with upregulation of GPX4.491
Toxin-mediated kidney toxicity
SB431542 and SB505124, ALK4/5 kinase inhibitors, suppress cadmium-induced HK-2 cell death.494 SIS3, a selective inhibitor of TGFβ1-dependent Smad3 phosphorylation, attenuates cadmium-induced cell death.494
Folic acid-induced kidney injury
Roxadustat (FG-4592) ameliorates folic acid-induced kidney injury by repressing ferroptosis via Akt/GSK-3β-mediated Nrf2 activation.599
Acute kidney injury
Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis through a reduction in p53 K382 acetylation via upregulation of SIRT1 expression.600 Dihydromyricetin attenuates cisplatin-induced AKI by inhibiting ferroptosis.601 Myo-inositol ameliorates cisplatin-induced AKI by inhibiting ferroptosis through the promotion of CHIP-mediated ubiquitination of NOX4.602
Diabetic kidney disease
Dapagliflozin ameliorates tubular injury by inhibiting ferroptosis through stabilization of SLC40A1 by reducing its ubiquitin-mediated degradation.603 Ginkgolide B alleviates DKD by inhibiting ferroptosis through inhibition of GPX4 ubiquitination.604 Schisandrin A attenuates ferroptosis and pyroptosis in DKD through attenuation of mitochondrial damage via AdipoR1 ubiquitination.605
Adriamycin-induced renal damage
Astragaloside IV attenuates adriamycin-induced renal damage by inhibiting ferroptosis through activation of PI3K/Akt and Nrf2.606
Concluding remarks and future perspectives
Epigenetic modifications and PTMs are essential for the maintenance of physical homeostasis and are implicated in the pathology of a variety of diseases. Accumulating evidence indicates that epigenetic modifications and PTMs play vital roles in regulating ferroptosis by controlling the expression of ferroptosis-associated genes in cancer, NSDs, CVDs, liver diseases, lung diseases, and kidney diseases. The present review highlights the crucial roles of epigenetic modifications and PTMs regulating ferroptosis in tumorigenesis and the genesis of NSDs, CVDs, liver diseases, lung diseases, and kidney diseases and clarified the regulatory interactions between epigenetic modifications and PTMs regulating ferroptosis in the genesis of these diseases. However, epigenetic modification-mediated regulation of ferroptosis in cancers is an emerging field and is still in its infancy. It is crucial to determine whether other epigenetic regulators play a role in regulating ferroptosis in cancers. First, in addition to ubiquitination, phosphorylation, acetylation, SUMOylation, O-GlcNAcylation, methylation, and ncRNA regulation, whether other epigenetic modifications, including ISGylation and lactylation, are involved in regulating ferroptosis remains unclear. Second, specific epigenetic modifications and PTMs regulate ferroptosis in specific cancers, NSDs, CVDs, liver diseases, lung diseases, and kidney diseases, and whether these epigenetic regulatory mechanisms are actually specific in other types of cancers remains an open question for future investigation. Third, epigenetic modifications and PTMs of targets that induce or inhibit ferroptosis have been identified, and dysregulated epigenetic modifications and PTMs can be feasibly targeted by small molecule compounds. Epigenetic drugs have exhibited viable therapeutic potential for NSDs,607,608,609,610 CVDs,611,612,613,614,615 liver diseases,616,617 lung diseases,618,619,620 and kidney diseases621,622,623 in preclinical and clinical trials. Epigenetic drugs targeting epigenetic regulators through modulation of epigenetic mechanisms have been applied and clinically translated for the treatment of hematological malignancies, greatly contributing to the development of antitumor drugs.118,124,624 Epigenetic drugs were found to exhibit viable therapeutic potential for solid tumors in preclinical and clinical trials.118,625 Combinations of epigenetic drugs with other therapies are being tested in preclinical research and clinical trials for the treatment of solid tumors.626,627 According to previous studies on clinical epigenetic drugs for hematological malignancies and studies that have revealed key roles of ferroptosis in mediating antitumor activity, we believe that epigenetic drugs targeting epigenetic regulators and ferroptosis could erase the roadmap to cancer. However, much remains to be done before the practical application of these treatment modalities, and inevitable challenges remain and need further clinical investigation. Herein, we highlight in detail the current efforts to translate this knowledge into clinical benefit for patients. Fourth, whether epigenetic modifications affect multiple ferroptosis regulators and how these different epigenetic modifications and PTMs cooperate with diverse signaling pathways to control the susceptibility of cancer cells to ferroptosis remain unclear. The epigenetic modification network of ferroptosis needs extensive investigation. Fifth, although ferroptosis has recently been confirmed to play a vital role in tumorigenesis and in the genesis of NSDs, CVDs, liver diseases, lung diseases, and kidney diseases, the detailed mechanisms related to ferroptosis and the molecular pathways involved remain open topics for future investigation. Moreover, the mechanisms by which epigenetic modifications and PTM events control the expression of ferroptosis-related genes in cancers, NSDs, CVDs, liver diseases, lung diseases, and kidney diseases remain incompletely elucidated. Determining whether mechanisms of novel epigenetic modifications and PTMs are actually specific in different diseases and cell types needs more systematic and comprehensive investigation.
In conclusion, epigenetic modifications and PTMs play fundamental roles in diseases, such as cancers, NSDs, CVDs, liver diseases, lung diseases, and kidney diseases. More studies on the effects of epigenetic modifications and PTMs on ferroptosis will ensure a better understanding of the pathogenesis of these diseases and identify novel paradigms for their treatment.
References
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Wang, Y. et al. Overcoming cancer chemotherapy resistance by the induction of ferroptosis. Drug Resist. Updat. 66, 100916 (2023).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Chen, X., Li, J., Kang, R., Klionsky, D. J. & Tang, D. Ferroptosis: machinery and regulation. Autophagy 17, 2054–2081 (2021).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Cavalli, G. & Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499 (2019).
Cao, J. & Yan, Q. Cancer epigenetics, tumor immunity, and immunotherapy. Trends Cancer 6, 580–592 (2020).
Wang, N., Ma, T. & Yu, B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct. Target Ther. 8, 69 (2023).
Wu, X. et al. Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. Signal Transduct. Target Ther. 8, 220 (2023).
Zhong, Q. et al. Protein posttranslational modifications in health and diseases: functions, regulatory mechanisms, and therapeutic implications. MedComm 4, e261 (2023).
Lee, J. & Roh, J. L. Epigenetic modulation of ferroptosis in cancer: identifying epigenetic targets for novel anticancer therapy. Cell Oncol. https://doi.org/10.1007/s13402-023-00840-7 (2023).
Wu, Y. et al. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Mol. Cancer 19, 39 (2020).
Hao, M., Jiang, Y., Zhang, Y., Yang, X. & Han, J. Ferroptosis regulation by methylation in cancer. Biochim. Biophys. Acta Rev. Cancer 1878, 188972 (2023).
Lan, T. et al. Epigenetic regulation of ferroptosis in central nervous system diseases. Mol. Neurobiol. 60, 3584–3599 (2023).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ. 28, 2843–2856 (2021).
Wang, Y., Wu, S., Li, Q., Sun, H. & Wang, H. Pharmacological inhibition of ferroptosis as a therapeutic target for neurodegenerative diseases and strokes. Adv. Sci. 10, e2300325 (2023).
Ou, M. et al. Role and mechanism of ferroptosis in neurological diseases. Mol. Metab. 61, 101502 (2022).
Lei, J. et al. Insight into the role of ferroptosis in non-neoplastic neurological diseases. Front. Cell Neurosci. 14, 231 (2020).
Ren, J. X., Sun, X., Yan, X. L., Guo, Z. N. & Yang, Y. Ferroptosis in neurological diseases. Front. Cell Neurosci. 14, 218 (2020).
Yao, M. Y. et al. Role of ferroptosis in neurological diseases. Neurosci. Lett. 747, 135614 (2021).
Li, N. et al. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol. Res. 166, 105466 (2021).
Fang, X., Ardehali, H., Min, J. & Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 20, 7–23 (2023).
Wu, X., Li, Y., Zhang, S. & Zhou, X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11, 3052–3059 (2021).
Yu, Y. et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 7, 193 (2021).
Guo, Y. et al. Roles of ferroptosis in cardiovascular diseases. Front. Cardiovasc. Med. 9, 911564 (2022).
Guo, G., Yang, W., Sun, C. & Wang, X. Dissecting the potential role of ferroptosis in liver diseases: an updated review. Free Radic. Res. 57, 282–293 (2023).
Wang, X., Zhou, Y., Min, J. & Wang, F. Zooming in and out of ferroptosis in human disease. Front. Med. 17, 173–206 (2023).
Yang, L., Cao, L. M., Zhang, X. J. & Chu, B. Targeting ferroptosis as a vulnerability in pulmonary diseases. Cell Death Dis. 13, 649 (2022).
Li, Y., Yang, Y. & Yang, Y. Multifaceted roles of ferroptosis in lung diseases. Front. Mol. Biosci. 9, 919187 (2022).
Yu, S. et al. Recent progress of ferroptosis in lung diseases. Front. Cell Dev. Biol. 9, 789517 (2021).
Xu, W. et al. Role of ferroptosis in lung diseases. J. Inflamm. Res. 14, 2079–2090 (2021).
Guo, R. et al. The road from AKI to CKD: molecular mechanisms and therapeutic targets of ferroptosis. Cell Death Dis. 14, 426 (2023).
Lei, G., Mao, C., Yan, Y., Zhuang, L. & Gan, B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 12, 836–857 (2021).
Guo, J. et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res Treat. 50, 445–460 (2018).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 18, 280–296 (2021).
Zhang, C., Liu, X., Jin, S., Chen, Y. & Guo, R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol. Cancer 21, 47 (2022).
Jin, Y., Ren, L., Jing, X. & Wang, H. Targeting ferroptosis as novel therapeutic approaches for epilepsy. Front Pharm. 14, 1185071 (2023).
Zhao, J., Wan, X. N., Zhu, J. P., Liu, Q. C. & Gan, L. LncRNA NEAT1 promoted MPP+-induced ferroptosis via regulating miR-150-5p/BAP1 pathway in SK-N-SH cells. Acta Neurobiol. Exp. 82, 226–236 (2022).
Yin, X. et al. Ferroptosis, a new insight into acute lung injury. Front. Pharm. 12, 709538 (2021).
Huo, L., Liu, C., Yuan, Y., Liu, X. & Cao, Q. Pharmacological inhibition of ferroptosis as a therapeutic target for sepsis-associated organ damage. Eur. J. Med. Chem. 257, 115438 (2023).
Hosohata, K., Harnsirikarn, T. & Chokesuwattanaskul, S. Ferroptosis: a potential therapeutic target in acute kidney injury. Int. J. Mol. Sci. 23, 6583 (2022).
Ni, L., Yuan, C. & Wu, X. Targeting ferroptosis in acute kidney injury. Cell Death Dis. 13, 182 (2022).
Ursini, F., Maiorino, M., Valente, M., Ferri, L. & Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710, 197–211 (1982).
Flohé, L. Looking back at the early stages of redox biology. Antioxidants. 9, 1254 (2020).
EAGLE, H. Nutrition needs of mammalian cells in tissue culture. Science 122, 501–514 (1955).
Mitchell, J. R., Jollow, D. J., Potter, W. Z., Gillette, J. R. & Brodie, B. B. Acetaminophen-induced hepatic necrosis IV. Protective role glutathione. J. Pharmacol. Exp. Ther. 187, 211–217 (1973).
Bannai, S., Tsukeda, H. & Okumura, H. Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem. Biophys. Res. Commun. 74, 1582–1588 (1977).
Tang, D. & Kroemer, G. Ferroptosis Curr. Biol. 30, R1292–R1297 (2020).
Chen, X., Kang, R., Kroemer, G. & Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 218, e20210518 (2021).
Sun, Y. et al. Mechanisms of ferroptosis and emerging links to the pathology of neurodegenerative diseases. Front Aging Neurosci. 14, 904152 (2022).
Kraft, V. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764.e22 (2023).
Kajarabille, N. & Latunde-Dada, G. O. Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J. Mol. Sci. 20, 4968 (2019).
Dodson, M., Castro-Portuguez, R. & Zhang, D. D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 23, 101107 (2019).
Qu, Z., Sun, J., Zhang, W., Yu, J. & Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic. Biol. Med. 159, 87–102 (2020).
Kerins, M. J. & Ooi, A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid. Redox Signal. 29, 1756–1773 (2018).
Lane, D., Metselaar, B., Greenough, M., Bush, A. I. & Ayton, S. J. Ferroptosis and NRF2: an emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays Biochem. 65, 925–940 (2021).
Cuadrado, A. et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18, 295–317 (2019).
Cuadrado, A. et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 70, 348–383 (2018).
Shakya, A., McKee, N. W., Dodson, M., Chapman, E. & Zhang, D. D. Anti-ferroptotic effects of Nrf2: beyond the antioxidant response. Mol. Cells 46, 165–175 (2023).
Nishizawa, H., Yamanaka, M. & Igarashi, K. Ferroptosis: regulation by competition between NRF2 and BACH1 and propagation of the death signal. FEBS J. 290, 1688–1704 (2023).
Lu, J., Zhao, Y., Liu, M., Lu, J. & Guan, S. Toward improved human health: Nrf2 plays a critical role in regulating ferroptosis. Food Funct. 12, 9583–9606 (2021).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Dixon, S. J. & Pratt, D. A. Ferroptosis: a flexible constellation of related biochemical mechanisms. Mol. Cell. 83, 1030–1042 (2023).
Hadian, K. & Stockwell, B. R. SnapShot: ferroptosis. Cell 181, 1188–1188.e1 (2020).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Zhang, H. L. et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 24, 88–98 (2022).
Shah, R., Shchepinov, M. S. & Pratt, D. A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018).
Gaschler, M. M. & Stockwell, B. R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482, 419–425 (2017).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Koppula, P., Zhuang, L. & Gan, B. Cytochrome P450 reductase (POR) as a ferroptosis fuel. Protein Cell 12, 675–679 (2021).
Yan, B. et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell. 81, 355–369.e10 (2021).
Zou, Y. et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 16, 302–309 (2020).
Wenzel, S. E. et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171, 628–641.e26 (2017).
Ge, C. et al. Emerging mechanisms and disease implications of ferroptosis: potential applications of natural products. Front. Cell Dev. Biol. 9, 774957 (2021).
Wang, Y. et al. Emerging mechanisms and targeted therapy of ferroptosis in neurological diseases and neuro-oncology. Int. J. Biol. Sci. 18, 4260–4274 (2022).
David, S., Jhelum, P., Ryan, F., Jeong, S. Y. & Kroner, A. Dysregulation of iron homeostasis in the central nervous system and the role of ferroptosis in neurodegenerative disorders. Antioxid. Redox Signal. 37, 150–170 (2022).
Kakhlon, O. & Cabantchik, Z. I. The labile iron pool: characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 33, 1037–1046 (2002).
Chen, X., Yu, C., Kang, R. & Tang, D. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 8, 590226 (2020).
Arosio, P., Ingrassia, R. & Cavadini, P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta 1790, 589–599 (2009).
Philpott, C. C. The flux of iron through ferritin in erythrocyte development. Curr. Opin. Hematol. 25, 183–188 (2018).
Lin, Q., Hou, S., Dai, Y., Jiang, N. & Lin, Y. Monascin exhibits neuroprotective effects in rotenone model of Parkinson’s disease via antioxidation and anti-neuroinflammation. Neuroreport 31, 637–643 (2020).
Gao, M. et al. Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032 (2016).
Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428 (2016).
Liu, M. Z. et al. The critical role of ferritinophagy in human disease. Front. Pharm. 13, 933732 (2022).
Mancias, J. D., Wang, X., Gygi, S. P., Harper, J. W. & Kimmelman, A. C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109 (2014).
Kremer, D. M. et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat. Commun. 12, 4860 (2021).
Sato, H., Tamba, M., Ishii, T. & Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274, 11455–11458 (1999).
Koppula, P., Zhuang, L. & Gan, B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12, 599–620 (2021).
Koppula, P., Zhang, Y., Zhuang, L. & Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 38, 12 (2018).
Brigelius-Flohé, R. & Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta. 1830, 3289–3303 (2013).
Brigelius-Flohé, R. & Flohé, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 33, 498–516 (2020).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422.e21 (2018).
Forcina, G. C. & Dixon, S. J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 19, e1800311 (2019).
Seibt, T. M., Proneth, B. & Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 133, 144–152 (2019).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Cui, C., Yang, F. & Li, Q. Post-translational modification of GPX4 is a promising target for treating ferroptosis-related diseases. Front. Mol. Biosci. 9, 901565 (2022).
Eaton, J. K. et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 16, 497–506 (2020).
Susin, S. A. et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397, 441–446 (1999).
Dai, E. et al. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun. 523, 966–971 (2020).
Pedrera, L. et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 28, 1644–1657 (2021).
Hogg, S. J., Beavis, P. A., Dawson, M. A. & Johnstone, R. W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 19, 776–800 (2020).
Dai, Z., Ramesh, V. & Locasale, J. W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 21, 737–753 (2020).
Chan, J. C. & Maze, I. Nothing is yet set in (Hi)stone: novel post-translational modifications regulating chromatin function. Trends Biochem. Sci. 45, 829–844 (2020).
Li, X., Egervari, G., Wang, Y., Berger, S. L. & Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 19, 563–578 (2018).
Park, J. W. & Han, J. W. Targeting epigenetics for cancer therapy. Arch. Pharm. Res. 42, 159–170 (2019).
Cheng, Y. et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 4, 62 (2019).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Mohammad, H. P., Barbash, O. & Creasy, C. L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat. Med. 25, 403–418 (2019).
Roulois, D. et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973 (2015).
Deblois, G. et al. Epigenetic switch-induced viral mimicry evasion in chemotherapy-resistant breast. Cancer Cancer Discov. 10, 1312–1329 (2020).
Shu, F. et al. Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduct. Target Ther. 8, 32 (2023).
Garcia-Martinez, L., Zhang, Y., Nakata, Y., Chan, H. L. & Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 12, 1786 (2021).
Ling, C. & Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 29, 1028–1044 (2019).
Cockram, P. E. et al. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 28, 591–605 (2021).
Liu, J. et al. Targeting the ubiquitination/deubiquitination process to regulate immune checkpoint pathways. Signal Transduct. Target Ther. 6, 28 (2021).
Chen, Q. et al. SOCS2-enhanced ubiquitination of SLC7A11 promotes ferroptosis and radiosensitization in hepatocellular carcinoma. Cell Death Differ. 30, 137–151 (2023).
Liu, Y. et al. PCDHB14 promotes ferroptosis and is a novel tumor suppressor in hepatocellular carcinoma. Oncogene 41, 3570–3583 (2022).
Sun, J. et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol. 41, 101942 (2021).
Guo, S. et al. TRIB2 modulates proteasome function to reduce ubiquitin stability and protect liver cancer cells against oxidative stress. Cell Death Dis. 12, 42 (2021).
Guo, S. et al. TRIB2 desensitizes ferroptosis via βTrCP-mediated TFRC ubiquitiantion in liver cancer cells. Cell Death Discov. 7, 196 (2021).
Yuan, J. et al. HDLBP-stabilized lncFAL inhibits ferroptosis vulnerability by diminishing Trim69-dependent FSP1 degradation in hepatocellular carcinoma. Redox Biol. 58, 102546 (2022).
Li, D. et al. Silencing TRPM2 enhanced erastin- and RSL3-induced ferroptosis in gastric cancer cells through destabilizing HIF-1α and Nrf2 proteins. Cytotechnology 74, 559–577 (2022).
Huang, G. et al. The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int. J. Biol. Sci. 18, 1415–1433 (2022).
Li, D. et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene 42, 83–98 (2023).
Zhang, H. et al. CAF-secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer 19, 43 (2020).
Han, Y. et al. Long noncoding RNA LINC00239 inhibits ferroptosis in colorectal cancer by binding to Keap1 to stabilize Nrf2. Cell Death Dis. 13, 742 (2022).
Gao, Y. et al. Knockdown of RRM1 in tumor cells promotes radio-/chemotherapy induced ferroptosis by regulating p53 ubiquitination and p21-GPX4 signaling axis. Cell Death Discov. 8, 343 (2022).
Zhu, Z. et al. FBXO31 sensitizes cancer stem cells-like cells to cisplatin by promoting ferroptosis and facilitating proteasomal degradation of GPX4 in cholangiocarcinoma. Liver Int. 42, 2871–2888 (2022).
Zeng, C. et al. SHARPIN promotes cell proliferation of cholangiocarcinoma and inhibits ferroptosis via p53/SLC7A11/GPX4 signaling. Cancer Sci. 113, 3766–3775 (2022).
Wang, Y. et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 20, e47563 (2019).
Zhang, Y. et al. TRIM6 reduces ferroptosis and chemosensitivity by targeting SLC1A5 in lung cancer. Oxid. Med Cell Longev. 2023, 9808100 (2023).
Tang, Z. et al. Deubiquitinase USP35 modulates ferroptosis in lung cancer via targeting ferroportin. Clin. Transl. Med. 11, e390 (2021).
Meng, C. et al. The deubiquitinase USP11 regulates cell proliferation and ferroptotic cell death via stabilization of NRF2 USP11 deubiquitinates and stabilizes NRF2. Oncogene 40, 1706–1720 (2021).
Li, K. et al. TRIM7 modulates NCOA4-mediated ferritinophagy and ferroptosis in glioblastoma cells. Redox Biol. 56, 102451 (2022).
Sun, Q. et al. Rho family GTPase 1 (RND1), a novel regulator of p53, enhances ferroptosis in glioblastoma. Cell Biosci. 12, 53 (2022).
Jiang, Y. et al. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J. Exp. Clin. Cancer Res. 41, 307 (2022).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
Liu, L. et al. ESR1 inhibits ionizing radiation-induced ferroptosis in breast cancer cells via the NEDD4L/CD71 pathway. Arch. Biochem. Biophys. 725, 109299 (2022).
Wang, X. et al. MTHFR inhibits TRC8-mediated HMOX1 ubiquitination and regulates ferroptosis in ovarian cancer. Clin. Transl. Med. 12, e1013 (2022).
Wang, Y., Wang, S. & Zhang, W. HRD1 functions as a tumor suppressor in ovarian cancer by facilitating ubiquitination-dependent SLC7A11 degradation. Cell Cycle 22, 1116–1126 (2023).
Shen, L. et al. PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder cancer. Int. J. Biol. Sci. 18, 5459–5474 (2022).
Jin, L. & Tong, L. PAQR3 inhibits proliferation and aggravates ferroptosis in acute lymphoblastic leukemia through modulation Nrf2 stability. Immun. Inflamm. Dis. 9, 827–839 (2021).
Zhu, T., Liu, B., Wu, D., Xu, G. & Fan, Y. Autophagy regulates VDAC3 ubiquitination by FBXW7 to promote erastin-induced ferroptosis in acute lymphoblastic leukemia. Front. Cell Dev. Biol. 9, 740884 (2021).
Yang, Y. et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 11, 433 (2020).
Li, Y. et al. Inhibition of CISD2 promotes ferroptosis through ferritinophagy-mediated ferritin turnover and regulation of p62-Keap1-NRF2 pathway. Cell. Mol. Biol. Lett. 27, 81 (2022).
Sugiura, M. et al. Epigenetic modifications in prostate cancer. Int. J. Urol. 28, 140–149 (2021).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Pang, K. et al. Role of protein phosphorylation in cell signaling, disease, and the intervention therapy. MedComm 3, e175 (2022).
Wang, Y. et al. Epigenetic modification of m(6)A regulator proteins in cancer. Mol. Cancer 22, 102 (2023).
Song, X. et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system xc- activity. Curr. Biol. 28, 2388–2399.e5 (2018).
Yang, C., Zhang, Y., Lin, S., Liu, Y. & Li, W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging 13, 13515–13534 (2021).
Wu, K. et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat. Cell Biol. 25, 714–725 (2023).
Yang, Y. et al. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 20, 587 (2020).
Ding, C. C. et al. MESH1 is a cytosolic NADPH phosphatase that regulates ferroptosis. Nat. Metab. 2, 270–277 (2020).
Lin, C. C. et al. The regulation of ferroptosis by MESH1 through the activation of the integrative stress response. Cell Death Dis. 12, 727 (2021).
Lin, C. C. et al. DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene 40, 2018–2034 (2021).
Chen, M. S. et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 8, 114588–114602 (2017).
Zhang, X. et al. Endogenous glutamate determines ferroptosis sensitivity via ADCY10-dependent YAP suppression in lung adenocarcinoma. Theranostics 11, 5650–5674 (2021).
Sun, X. et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34, 5617–5625 (2015).
Shang, S., Liu, J. & Hua, F. Protein acylation: mechanisms, biological functions and therapeutic targets. Signal Transduct. Target Ther. 7, 396 (2022).
Phillips, D. M. The presence of acetyl groups of histones. Biochem. J. 87, 258–263 (1963).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Wang, S. J. et al. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 17, 366–373 (2016).
Chen, L. et al. GINS4 suppresses ferroptosis by antagonizing p53 acetylation with Snail. Proc. Natl Acad. Sci. USA 120, e2219585120 (2023).
Cui, X. et al. HMGCL-induced β-hydroxybutyrate production attenuates hepatocellular carcinoma via DPP4-mediated ferroptosis susceptibility. Hepatol. Int. 17, 377–392 (2023).
Zheng, X. et al. N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA. Cancer Commun. 42, 1347–1366 (2022).
Liu, Y. et al. KAT6B may be applied as a potential therapeutic target for glioma. J. Oncol. 2022, 2500092 (2022).
Kon, N. et al. mTOR inhibition acts as an unexpected checkpoint in p53-mediated tumor suppression. Genes Dev. 35, 59–64 (2021).
AMBLER, R. P. & REES, M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature 184, 56–57 (1959).
Bhat, K. P., Ümit Kaniskan, H., Jin, J. & Gozani, O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat. Rev. Drug Discov. 20, 265–286 (2021).
MURRAY, K. The occurrence of epsilon-n-methyl lysine in histones. Biochemistry 3, 10–15 (1964).
Murn, J. & Shi, Y. The winding path of protein methylation research: milestones and new frontiers. Nat. Rev. Mol. Cell Biol. 18, 517–527 (2017).
Michalak, E. M., Burr, M. L., Bannister, A. J. & Dawson, M. A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 20, 573–589 (2019).
Bedford, M. T. & Richard, S. Arginine methylation an emerging regulator of protein function. Mol. Cell. 18, 263–272 (2005).
Smith, B. C. & Denu, J. M. Chemical mechanisms of histone lysine and arginine modifications. Biochim. Biophys. Acta 1789, 45–57 (2009).
Hamamoto, R., Saloura, V. & Nakamura, Y. Critical roles of non-histone protein lysine methylation in human tumorigenesis. Nat. Rev. Cancer 15, 110–124 (2015).
Rodríguez-Paredes, M. & Lyko, F. The importance of non-histone protein methylation in cancer therapy. Nat. Rev. Mol. Cell Biol. 20, 569–570 (2019).
Lee, J. Y. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl Acad. Sci. USA. 117, 32433–32442 (2020).
Zou, J. et al. Methylation drives SLC2A1 transcription and ferroptosis process decreasing autophagy pressure in colon cancer. J. Oncol. 2022, 9077424 (2022).
Zhang, X. et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 235, 3425–3437 (2020).
Wang, Z. et al. CREB stimulates GPX4 transcription to inhibit ferroptosis in lung adenocarcinoma. Oncol. Rep. 45, 88 (2021).
Deng, H. et al. SET7 methylates the deubiquitinase OTUB1 at Lys (122) to impair its binding to E2 enzyme UBC13 and relieve its suppressive role on ferroptosis. J. Biol. Chem. 299, 103054 (2023).
Gong, Q. et al. CX3CL1 promotes cell sensitivity to ferroptosis and is associated with the tumor microenvironment in clear cell renal cell carcinoma. BMC Cancer 22, 1184 (2022).
Yang, J. et al. Functional deficiency of succinate dehydrogenase promotes tumorigenesis and development of clear cell renal cell carcinoma through weakening of ferroptosis. Bioengineered 13, 11187–11207 (2022).
Pontel, L. B. et al. Acute lymphoblastic leukemia necessitates GSH-dependent ferroptosis defenses to overcome FSP1-epigenetic silencing. Redox Biol. 55, 102408 (2022).
Logie, E. et al. Ferroptosis induction in multiple myeloma cells triggers DNA methylation and histone modification changes associated with cellular senescence. Int. J. Mol. Sci. 22, 12234 (2021).
Wang, Y. et al. Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11. FEBS Open Bio 10, 637–643 (2020).
Chen, M., Jiang, Y. & Sun, Y. KDM4A-mediated histone demethylation of SLC7A11 inhibits cell ferroptosis in osteosarcoma. Biochem. Biophys. Res. Commun. 550, 77–83 (2021).
Yang, X. & Qian, K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 18, 452–465 (2017).
Fehl, C. & Hanover, J. A. Tools, tactics and objectives to interrogate cellular roles of O-GlcNAc in disease. Nat. Chem. Biol. 18, 8–17 (2022).
Slawson, C. & Hart, G. W. O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Cancer 11, 678–684 (2011).
Ferreira, J. A. et al. Mechanisms of cisplatin resistance and targeting of cancer stem cells: adding glycosylation to the equation. Drug Resist. Updat. 24, 34–54 (2016).
Chen, Y. et al. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell. Signal. 63, 109384 (2019).
Zhu, G. et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. 7, 83 (2021).
Wang, X. et al. O-GlcNAcylation of ZEB1 facilitated mesenchymal pancreatic cancer cell ferroptosis. Int. J. Biol. Sci. 18, 4135–4150 (2022).
Li, H. W. et al. GALNT14 regulates ferroptosis and apoptosis of ovarian cancer through the EGFR/mTOR pathway. Future Oncol. 18, 149–161 (2022).
Liu, P. et al. Inhibition of ALG3 stimulates cancer cell immunogenic ferroptosis to potentiate immunotherapy. Cell. Mol. Life Sci. 79, 352 (2022).
Yu, F. et al. Dynamic O-GlcNAcylation coordinates ferritinophagy and mitophagy to activate ferroptosis. Cell Discov. 8, 40 (2022).
Seeler, J. S. & Dejean, A. SUMO and the robustness of cancer. Nat. Rev. Cancer 17, 184–197 (2017).
Kroonen, J. S. & Vertegaal, A. Targeting SUMO signaling to wrestle cancer. Trends Cancer 7, 496–510 (2021).
Du, L., Liu, W. & Rosen, S. T. Targeting SUMOylation in cancer. Curr. Opin. Oncol. 33, 520–525 (2021).
Gao, C. et al. SENP1 inhibition suppresses the growth of lung cancer cells through activation of A20-mediated ferroptosis. Ann. Transl. Med. 10, 224 (2022).
Zhang, K. et al. Heat shock protein 27 deficiency promotes ferrous ion absorption and enhances acyl-Coenzyme A synthetase long-chain family member 4 stability to promote glioblastoma cell ferroptosis. Cancer Cell Int. 23, 5 (2023).
Song, Z., Jia, G., Ma, P. & Cang, S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 276, 119399 (2021).
Ma, L. et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. 38, 101801 (2021).
Ma, L. et al. Targeting SLC3A2 subunit of system X(C)(-) is essential for m(6)A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic. Biol. Med. 168, 25–43 (2021).
Xu, X. et al. IGF2BP3 is an essential N(6)-methyladenosine biotarget for suppressing ferroptosis in lung adenocarcinoma cells. Mater. Today Bio 17, 100503 (2022).
Xu, Y. et al. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m(6)A modification. Cancer Cell Int. 22, 11 (2022).
Lu, Z. et al. IGF2BP3-NRF2 axis regulates ferroptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 627, 103–110 (2022).
Liu, L. et al. The N6-methyladenosine modification enhances ferroptosis resistance through inhibiting SLC7A11 mRNA deadenylation in hepatoblastoma. Clin. Transl. Med. 12, e778 (2022).
Li, W., Huang, G., Wei, J., Cao, H. & Jiang, G. ALKBH5 inhibits thyroid cancer progression by promoting ferroptosis through TIAM1-Nrf2/HO-1 axis. Mol. Cell. Biochem. (2022).
Ji, F. H., Fu, X. H., Li, G. Q., He, Q. & Qiu, X. G. FTO prevents thyroid cancer progression by SLC7A11 m6A methylation in a ferroptosis-dependent manner. Front. Endocrinol. 13, 857765 (2022).
Ye, F., Wu, J. & Zhang, F. METTL16 epigenetically enhances GPX4 expression via m6A modification to promote breast cancer progression by inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 638, 1–6 (2023).
Zou, Y. et al. N6-methyladenosine regulated FGFR4 attenuates ferroptotic cell death in recalcitrant HER2-positive breast cancer. Nat. Commun. 13, 2672 (2022).
Sun, S. et al. RNA binding protein NKAP protects glioblastoma cells from ferroptosis by promoting SLC7A11 mRNA splicing in an m(6)A-dependent manner. Cell Death Dis. 13, 73 (2022).
Huang, W. M. et al. m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis. Transl. Oncol. 27, 101576 (2023).
Ye, J. et al. RNA demethylase ALKBH5 regulates hypopharyngeal squamous cell carcinoma ferroptosis by posttranscriptionally activating NFE2L2/NRF2 in an m(6) A-IGF2BP2-dependent manner. J. Clin. Lab. Anal. 36, e24514 (2022).
Yang, H. et al. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J. Adv. Res. 37, 91–106 (2022).
Luo, Y. et al. Regulation of ferroptosis by non-coding RNAs in the development and treatment of cancer (Review). Oncol. Rep. 45, 29–48 (2021).
Xie, B. & Guo, Y. Molecular mechanism of cell ferroptosis and research progress in regulation of ferroptosis by noncoding RNAs in tumor cells. Cell Death Discov. 7, 101 (2021).
Balihodzic, A. et al. Non-coding RNAs and ferroptosis: potential implications for cancer therapy. Cell Death Differ. 29, 1094–1106 (2022).
Zuo, Y. B. et al. Ferroptosis in cancer progression: role of noncoding RNAs. Int. J. Biol. Sci. 18, 1829–1843 (2022).
Cookson, M. R. The biochemistry of Parkinson’s disease. Annu. Rev. Biochem. 74, 29–52 (2005).
Abeliovich, A. & Flint Beal, M. Parkinsonism genes: culprits and clues. J. Neurochem. 99, 1062–1072 (2006).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Wang, Y. et al. Pharmacological modulation of Nrf2/HO-1 signaling pathway as a therapeutic target of Parkinson’s disease. Front Pharm. 12, 757161 (2021).
Aarsland, D. et al. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 13, 217–231 (2017).
Li, K. et al. ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol. Res. 193, 106779 (2023).
Lin, Z. H. et al. Quercetin protects against MPP(+)/MPTP-induced dopaminergic neuron death in Parkinson’s disease by inhibiting ferroptosis. Oxid. Med. Cell Longev. 2022, 7769355 (2022).
Zhao, Y. et al. Proteomic analysis of protective effects of Dl-3-n-butylphthalide against mpp + -induced toxicity via downregulating P53 pathway in N2A Cells. Proteome Sci. 21, 1 (2023).
Yu, X. et al. Ketone body β-hydroxybutyric acid ameliorates dopaminergic neuron injury through modulating zinc finger protein 36/Acyl-CoA synthetase long-chain family member four signaling axis-mediated ferroptosis. Neuroscience 509, 157–172 (2022).
Hu, C. B. et al. DL-3-n-butylphthalide alleviates motor disturbance by suppressing ferroptosis in a rat model of Parkinson’s disease. Neural Regen. Res. 18, 194–199 (2023).
Sun, Y. et al. Activation of Atg7-dependent autophagy by a novel inhibitor of the Keap1-Nrf2 protein-protein interaction from Penthorum Chinese Pursh. attenuates 6-hydroxydopamine-induced ferroptosis in zebrafish and dopaminergic neurons. Food Funct. 13, 7885–7900 (2022).
Lee, W. J. et al. PPARδ activation mitigates 6-OHDA-induced neuronal damage by regulating intracellular iron levels. Antioxidants 11, 810 (2022).
Xi, J. et al. Hinokitiol functions as a ferroptosis inhibitor to confer neuroprotection. Free Radic. Biol. Med. 190, 202–215 (2022).
Wang, L. et al. The neuroprotective effects of paeoniflorin against MPP(+)-induced damage to dopaminergic neurons via the Akt/Nrf2/GPX4 pathway. J. Chem. Neuroanat. 122, 102103 (2022).
Liu, L., Yang, S. & Wang, H. α-Lipoic acid alleviates ferroptosis in the MPP(+) -induced PC12 cells via activating the PI3K/Akt/Nrf2 pathway. Cell Biol. Int. 45, 422–431 (2021).
Zeng, X. et al. Benefits of iron chelators in the treatment of Parkinson’s disease. Neurochem. Res. 46, 1239–1251 (2021).
Gutbier, S. et al. Design and evaluation of bi-functional iron chelators for protection of dopaminergic neurons from toxicants. Arch. Toxicol. 94, 3105–3123 (2020).
Shi, L. et al. Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging 12, 9515–9533 (2020).
Song, L. M. et al. Apoferritin improves motor deficits in MPTP-treated mice by regulating brain iron metabolism and ferroptosis. iScience 24, 102431 (2021).
Sun, Y. et al. Activation of p62-Keap1-Nrf2 pathway protects 6-Hydroxydopamine-induced ferroptosis in dopaminergic cells. Mol. Neurobiol. 57, 4628–4641 (2020).
Berry, T. M. & Moustafa, A. A. A novel treatment strategy to prevent Parkinson’s disease: focus on iron regulatory protein 1 (IRP1). Int. J. Neurosci. 133, 67–76 (2023).
Avcı, B. et al. Idebenone ameliorates rotenone-induced Parkinson’s disease in rats through decreasing lipid peroxidation. Neurochem. Res. 46, 513–522 (2021).
Mahoney-Sánchez, L. et al. Ferroptosis and its potential role in the physiopathology of Parkinson’s disease. Prog. Neurobiol. 196, 101890 (2021).
Dionísio, P. A., Amaral, J. D. & Rodrigues, C. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 67, 101263 (2021).
Thapa, K. et al. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 930, 175133 (2022).
Shibu, M. A., Bharath, M. & Velmurugan, B. K. Regulating inflammation associated ferroptosis - a treatment strategy for Parkinson disease. Curr. Med. Chem. 28, 6895–6914 (2021).
Wen, S., Aki, T., Unuma, K. & Uemura, K. Chemically induced models of Parkinson’s disease: history and perspectives for the involvement of ferroptosis. Front Cell Neurosci. 14, 581191 (2020).
Vitalakumar, D., Sharma, A. & Flora, S. Ferroptosis: a potential therapeutic target for neurodegenerative diseases. J. Biochem. Mol. Toxicol. 35, e22830 (2021).
Li, X. et al. miR-335 promotes ferroptosis by targeting ferritin heavy chain 1 in in vivo and in vitro models of Parkinson’s disease. Int. J. Mol. Med. 47, 61 (2021).
Gorelick, P. B. The global burden of stroke: persistent and disabling. Lancet Neurol. 18, 417–418 (2019).
Feigin, V. L. et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 379, 2429–2437 (2018).
Tuo, Q. Z., Zhang, S. T. & Lei, P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 42, 259–305 (2022).
Bu, Z. Q. et al. Emerging role of ferroptosis in the pathogenesis of ischemic stroke: a new therapeutic target. ASN Neuro 13, 17590914211037505 (2021).
Xu, Y. et al. Role of ferroptosis in stroke. Cell. Mol. Neurobiol. 43, 205–222 (2023).
Zhang, Y., Lu, X., Tai, B., Li, W. & Li, T. Ferroptosis and its multifaceted roles in cerebral stroke. Front Cell Neurosci. 15, 615372 (2021).
Liu, Y. et al. Ferroptosis: an emerging therapeutic target in stroke. J. Neurochem. 160, 64–73 (2022).
Lou, Y. et al. Ferroptosis: a new strategy for traditional Chinese medicine treatment of stroke. Biomed. Pharmacother. 156, 113806 (2022).
Yang, K. et al. The mechanism of ferroptosis regulating oxidative stress in ischemic stroke and the regulation mechanism of natural pharmacological active components. Biomed. Pharmacother. 154, 113611 (2022).
Guo, L. & Shi, L. Vitexin improves cerebral ischemia-reperfusion injury by attenuating oxidative injury and ferroptosis via Keap1/Nrf2/HO-1signaling. Neurochem. Res. 48, 980–995 (2023).
Liu, H. et al. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch. Biochem. Biophys. 734, 109488 (2023).
Zhan, S. et al. SATB1/SLC7A11/HO-1 axis ameliorates ferroptosis in neuron cells after ischemic stroke by danhong injection. Mol. Neurobiol. 60, 413–427 (2023).
Liu, X. et al. TUK9Ferrostatin-1 alleviates cerebral ischemia/reperfusion injury through activation of the AKT/GSK3β signaling pathway. Brain Res. Bull. 193, 146–157 (2023).
Gao, J. et al. Icariside II preconditioning evokes robust neuroprotection against ischemic stroke: targeting Nrf2 mediated by OXPHOS/NF-κB/ferroptosis pathway. Br. J. Pharmacol. 180, 308–329 (2023).
Guo, H. et al. Carthamin yellow improves cerebral ischemia-reperfusion injury by attenuating inflammation and ferroptosis in rats. Int. J. Mol. Med. 47, 52 (2021).
Guan, X. et al. Galangin attenuated cerebral ischemia-reperfusion injury by inhibition of ferroptosis through activating the SLC7A11/GPX4 axis in gerbils. Life Sci. 264, 118660 (2021).
Fu, C. et al. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 289, 115021 (2022).
Yan, N., Xu, Z., Qu, C. & Zhang, J. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int. Immunopharmacol. 98, 107844 (2021).
Shi, Y. et al. Selenium alleviates cerebral ischemia/reperfusion injury by regulating oxidative stress, mitochondrial fusion and ferroptosis. Neurochem. Res. 47, 2992–3002 (2022).
Guan, X. et al. The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sci. 235, 116795 (2019).
Chen, J., Ma, D., Bao, J., Zhang, Y. & Deng, G. Roots of astragalus propinquus schischkin regulate transmembrane iron transport and ferroptosis to improve cerebral ischemia-reperfusion injury. Evid. Based Complement Altern. Med 2022, 7410865 (2022).
Lan, B. et al. Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med. 18, 344–350 (2020).
Hu, Q. et al. β-Caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the NRF2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine 102, 154112 (2022).
Hu, M. et al. Dexmedetomidine exerts its protective effect on cerebral ischemia reperfusion injury in mice by inhibiting ferroptosis. Zhong nan da xue xue bao. Yi xue ban. = J. Cent. South Univ. Med. Sci. 47, 600–609 (2022).
Liu, W. et al. Edaravone ameliorates cerebral ischemia-reperfusion injury by downregulating ferroptosis via the Nrf2/FPN pathway in rats. Biol. Pharm. Bull. 45, 1269–1275 (2022).
Li, M. et al. Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem. Biol. Interact. 366, 110137 (2022).
Chen, G. et al. Hydroxysafflor yellow A and anhydrosafflor yellow B alleviate ferroptosis and parthanatos in PC12 cells injured by OGD/R. Free Radic. Biol. Med. 179, 1–10 (2022).
Hui, Z., Wang, S., Li, J., Wang, J. & Zhang, Z. Compound Tongluo Decoction inhibits endoplasmic reticulum stress-induced ferroptosis and promoted angiogenesis by activating the Sonic Hedgehog pathway in cerebral infarction. J. Ethnopharmacol. 283, 114634 (2022).
Zhu, H. et al. Resveratrol pretreatment protects neurons from oxygen-glucose deprivation/reoxygenation and ischemic injury through inhibiting ferroptosis. Biosci. Biotechnol. Biochem. 86, 704–716 (2022).
Mao, R. & Liu, H. Depletion of mmu_circ_0001751 (circular RNA Carm1) protects against acute cerebral infarction injuries by binding with microRNA-3098-3p to regulate acyl-CoA synthetase long-chain family member 4. Bioengineered 13, 4063–4075 (2022).
Lu, J., Xu, F. & Lu, H. LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 260, 118305 (2020).
Zhang, J. et al. Micro ribonucleic acid 27a aggravates ferroptosis during early ischemic stroke of rats through nuclear factor erythroid-2-related factor 2. Neuroscience 504, 10–20 (2022).
Zhu, L., Feng, Z., Zhang, J., Du, L. & Meng, A. MicroRNA-27a regulates ferroptosis through SLC7A11 to aggravate cerebral ischemia-reperfusion injury. Neurochem. Res. 48, 1370–1381 (2023).
Wang, Y. et al. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnol. 21, 109 (2023).
Fan, W. et al. GATA6 inhibits neuronal autophagy and ferroptosis in cerebral ischemia-reperfusion injury through a miR-193b/ATG7 axis-dependent mechanism. Neurochem. Res. 48, 2552–2567 (2023).
Chen, C. et al. Long noncoding RNA Meg3 mediates ferroptosis induced by oxygen and glucose deprivation combined with hyperglycemia in rat brain microvascular endothelial cells, through modulating the p53/GPX4 axis. Eur. J. Histochem. 65, 3224 (2021).
Du, Y. et al. Downregulation of ELAVL1 attenuates ferroptosis-induced neuronal impairment in rats with cerebral ischemia/reperfusion via reducing DNMT3B-dependent PINK1 methylation. Metab. Brain Dis. 37, 2763–2775 (2022).
Li, C. et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol. Res. 174, 105933 (2021).
Sanguigno, L. et al. Stroke by inducing HDAC9-dependent deacetylation of HIF-1 and Sp1, promotes TfR1 transcription and GPX4 reduction, thus determining ferroptotic neuronal death. Int. J. Biol. Sci. 19, 2695–2710 (2023).
Greenberg, S. M. et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American. Stroke Association. Stroke 53, e282–e361 (2022).
Liu, M. J., Zhao, X. C., Gong, H. S., You, Y. Q. & Li, J. Y. Dexmedetomidine prevents hemorrhagic brain injury by reducing damage induced by ferroptosis in mice. Neurosci. Lett. 788, 136842 (2022).
Wang, B. et al. Dexpramipexole attenuates white matter injury to facilitate locomotion and motor coordination recovery via reducing ferroptosis after intracerebral hemorrhage. Oxid. Med. Cell Longev. 2022, 6160701 (2022).
Zhou, Z. X. et al. Withaferin A inhibits ferroptosis and protects against intracerebral hemorrhage. Neural Regen. Res. 18, 1308–1315 (2023).
Duan, C. et al. Activation of the PPARγ prevents ferroptosis-induced neuronal loss in response to intracerebral hemorrhage through synergistic actions with the Nrf2. Front. Pharm. 13, 869300 (2022).
Wang, F. et al. Crocin alleviates intracerebral hemorrhage-induced neuronal ferroptosis by facilitating Nrf2 nuclear translocation. Neurotox. Res. 40, 596–604 (2022).
Zhang, Y., Zhang, X., Wee Yong, V. & Xue, M. Vildagliptin improves neurological function by inhibiting apoptosis and ferroptosis following intracerebral hemorrhage in mice. Neurosci. Lett. 776, 136579 (2022).
Peng, C. et al. Dauricine alleviated secondary brain injury after intracerebral hemorrhage by upregulating GPX4 expression and inhibiting ferroptosis of nerve cells. Eur. J. Pharmacol. 914, 174461 (2022).
Chang, C. F., Cho, S. & Wang, J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways.Ann. Clin.Transl. Neurol. 1, 258–271 (2014)..
Jin, Z. L. et al. Paeonol inhibits the progression of intracerebral haemorrhage by mediating the HOTAIR/UPF1/ACSL4 axis. ASN Neuro 13, 17590914211010647 (2021).
Zhao, H. et al. Isorhynchophylline relieves ferroptosis-induced nerve damage after intracerebral hemorrhage via miR-122-5p/TP53/SLC7A11 pathway. Neurochem. Res. 46, 1981–1994 (2021).
Han, R. et al. 20-HETE participates in intracerebral hemorrhage-induced acute injury by promoting cell ferroptosis. Front. Neurol. 12, 763419 (2021).
Yang, C. et al. Curcumin nanoparticles inhibiting ferroptosis for the enhanced treatment of intracerebral hemorrhage. Int. J. Nanomed. 16, 8049–8065 (2021).
Zhang, H. et al. Pyridoxal isonicotinoyl hydrazone improves neurological recovery by attenuating ferroptosis and inflammation in cerebral hemorrhagic mice. Biomed. Res. Int. 2021, 9916328 (2021).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279.e25 (2019).
Duan, L. et al. Baicalin inhibits ferroptosis in intracerebral hemorrhage. Front. Pharm. 12, 629379 (2021).
Karuppagounder, S. S. et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E(2) to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 84, 854–872 (2018).
Mo, Y. et al. Nanoparticles improved resveratrol brain delivery and its therapeutic efficacy against intracerebral hemorrhage. Nanoscale 13, 3827–3840 (2021).
Bai, Q., Liu, J. & Wang, G. Ferroptosis, a regulated neuronal cell death type after intracerebral hemorrhage. Front. Cell Neurosci. 14, 591874 (2020).
Ren, S., Chen, Y., Wang, L. & Wu, G. Neuronal ferroptosis after intracerebral hemorrhage. Front. Mol. Biosci. 9, 966478 (2022).
Wan, J., Ren, H. & Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 4, 93–95 (2019).
Derry, P. J. et al. The chemical basis of intracerebral hemorrhage and cell toxicity with contributions from eryptosis and ferroptosis. Front. Cell Neurosci. 14, 603043 (2020).
Zhou, S. Y. et al. Mechanism of ferroptosis and its relationships with other types of programmed cell death: insights for potential interventions after intracerebral hemorrhage. Front. Neurosci. 14, 589042 (2020).
Wei, Y. et al. Iron toxicity in intracerebral hemorrhage: physiopathological and therapeutic implications. Brain Res. Bull. 178, 144–154 (2022).
Pan, F., Xu, W., Ding, J. & Wang, C. Elucidating the progress and impact of ferroptosis in hemorrhagic stroke. Front. Cell Neurosci. 16, 1067570 (2022).
Cao, Y., Xiao, W., Liu, S. & Zeng, Y. Ferroptosis: underlying mechanism and the crosstalk with other modes of neuronal death after intracerebral hemorrhage. Front. Cell Neurosci. 17, 1080344 (2023).
Chen, B., Wang, H., Lv, C., Mao, C. & Cui, Y. Long non-coding RNA H19 protects against intracerebral hemorrhage injuries via regulating microRNA-106b-5p/acyl-CoA synthetase long chain family member 4 axis. Bioengineered 12, 4004–4015 (2021).
Li, Y. et al. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res. Ther. 11, 330 (2020).
Bao, W. D. et al. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell 19, e13235 (2020).
Kong, Y. et al. Acupuncture ameliorates neuronal cell death, inflammation, and ferroptosis and downregulated miR-23a-3p after intracerebral hemorrhage in rats. J. Mol. Neurosci. 71, 1863–1875 (2021).
Nie, Z., Tan, L., Niu, J. & Wang, B. The role of regulatory necrosis in traumatic brain injury. Front. Mol. Neurosci. 15, 1005422 (2022).
Rauchman, S. H. et al. Traumatic brain injury: mechanisms, manifestations, and visual sequelae. Front. Neurosci. 17, 1090672 (2023).
Maas, A. et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 21, 1004–1060 (2022).
Mollayeva, T., Mollayeva, S. & Colantonio, A. Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 14, 711–722 (2018).
Simon, D. W. et al. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 572 (2017).
Pang, Q. et al. Mechanism of ferroptosis and its relationships with other types of programmed cell death: insights for potential therapeutic benefits in traumatic brain injury. Oxid. Med. Cell Longev. 2022, 1274550 (2022).
Li, Q. S. & Jia, Y. J. Ferroptosis: a critical player and potential therapeutic target in traumatic brain injury and spinal cord injury. Neural Regen. Res. 18, 506–512 (2023).
Hu, X. et al. Progress in understanding ferroptosis and its targeting for therapeutic benefits in traumatic brain and spinal cord injuries. Front Cell Dev. Biol. 9, 705786 (2021).
Geng, Z. et al. Ferroptosis and traumatic brain injury. Brain Res. Bull. 172, 212–219 (2021).
Kenny, E. M. et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. CARE Med. 47, 410–418 (2019).
Xie, B. S. et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 25, 465–475 (2019).
Gao, Y. et al. Annexin A5 ameliorates traumatic brain injury-induced neuroinflammation and neuronal ferroptosis by modulating the NF-ĸB/HMGB1 and Nrf2/HO-1 pathways. Int. Immunopharmacol. 114, 109619 (2023).
Fang, J. et al. Ferroptosis in brain microvascular endothelial cells mediates blood-brain barrier disruption after traumatic brain injury. Biochem. Biophys. Res. Commun. 619, 34–41 (2022).
Rui, T., Li, Q., Song, S., Gao, Y. & Luo, C. Ferroptosis-relevant mechanisms and biomarkers for therapeutic interventions in traumatic brain injury. Histol. Histopathol. 35, 1105–1113 (2020).
Tang, S. et al. The role of iron, its metabolism and ferroptosis in traumatic brain injury. Front Cell Neurosci. 14, 590789 (2020).
Cheng, Y. et al. Ferristatin II, an iron uptake inhibitor, exerts neuroprotection against traumatic brain injury via suppressing ferroptosis. ACS Chem. Neurosci. 13, 664–675 (2022).
Qin, D. et al. Traumatic brain injury: ultrastructural features in neuronal ferroptosis, glial cell activation and polarization, and blood-brain barrier breakdown. Cells. 10, 1009 (2021).
Liu, H. et al. Tetrandrine ameliorates traumatic brain injury by regulating autophagy to reduce ferroptosis. Neurochem. Res. 47, 1574–1587 (2022).
Huang, L. et al. Polydatin alleviates traumatic brain injury: role of inhibiting ferroptosis. Biochem. Biophys. Res. Commun. 556, 149–155 (2021).
Cheng, Y. et al. TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic. Biol. Med. 194, 184–198 (2023).
Jia, H. et al. Deferoxamine ameliorates neurological dysfunction by inhibiting ferroptosis and neuroinflammation after traumatic brain injury. Brain Res. 1812, 148383 (2023).
Xiao, X. et al. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain 12, 78 (2019).
Zipser, C. M. et al. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 21, 659–670 (2022).
Freund, P. et al. MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol. 18, 1123–1135 (2019).
David, G. et al. Traumatic and nontraumatic spinal cord injury: pathological insights from neuroimaging. Nat. Rev. Neurol. 15, 718–731 (2019).
Ahuja, C. S. et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 3, 17018 (2017).
Anderson, M. A. et al. Natural and targeted circuit reorganization after spinal cord injury. Nat. Neurosci. 25, 1584–1596 (2022).
Bai, X. Y. et al. Ferroptosis is a new therapeutic target for spinal cord injury. Front. Neurosci. 17, 1136143 (2023).
Li, F. et al. Mechanism of ferroptosis and its role in spinal cord injury. Front. Neurol. 13, 926780 (2022).
Yu, Z., Cheng, X., Pan, W., Yu, C. & Duan, Y. The ferroptosis activity is associated with neurological recovery following chronic compressive spinal cord injury. Neural Regen. Res. 18, 2482–2488 (2023).
Wang, F. et al. miR-672-3p promotes functional recovery in rats with contusive spinal cord injury by inhibiting ferroptosis suppressor protein 1. Oxid. Med. Cell Longev. 2022, 6041612 (2022).
Shao, C., Chen, Y., Yang, T., Zhao, H. & Li, D. Mesenchymal stem cell derived exosomes suppress neuronal cell ferroptosis via lncGm36569/miR-5627-5p/FSP1 axis in acute spinal cord injury. Stem Cell Rev. Rep. 18, 1127–1142 (2022).
Ma, Z. et al. miR-6315 silencing protects against spinal cord injury through the Smo and anti-ferroptosis pathway. Biosci. Rep. 43, BSR20230030 (2023).
Wang, C. et al. USP7 regulates HMOX-1 via deubiquitination to suppress ferroptosis and ameliorate spinal cord injury in rats. Neurochem. Int. 168, 105554 (2023).
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Kuhlmann, T. et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 22, 78–88 (2023).
Faissner, S., Plemel, J. R., Gold, R. & Yong, V. W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922 (2019).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Krämer, J., Bar-Or, A., Turner, T. J. & Wiendl, H. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat. Rev. Neurol. 19, 289–304 (2023).
Fan, J. et al. Mesenchymal stem cell-derived exosomal microRNA-367-3p alleviates experimental autoimmune encephalomyelitis via inhibition of microglial ferroptosis by targeting EZH2. Biomed. Pharmacother. 162, 114593 (2023).
Duarte-Silva, E., Meuth, S. G. & Peixoto, C. A. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 14, 1137635 (2023).
White, A. R. Ferroptosis drives immune-mediated neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 20, 112–113 (2023).
Li, X. et al. Ferroptosis as a mechanism of oligodendrocyte loss and demyelination in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 373, 577995 (2022).
Luoqian, J. et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 19, 913–924 (2022).
Hu, C. L. et al. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurochem. 148, 426–439 (2019).
Rothammer, N. et al. G9a dictates neuronal vulnerability to inflammatory stress via transcriptional control of ferroptosis. Sci. Adv. 8, eabm5500 (2022).
Algoet, M. et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 33, 357–366 (2023).
Frank, A. et al. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac. Vasc. Anesth. 16, 123–132 (2012).
Ibáñez, B., Heusch, G., Ovize, M. & Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 65, 1454–1471 (2015).
Li, J. Y., Liu, S. Q., Yao, R. Q., Tian, Y. P. & Yao, Y. M. A novel insight into the fate of cardiomyocytes in ischemia-reperfusion injury: from iron metabolism to ferroptosis. Front. Cell Dev. Biol. 9, 799499 (2021).
Fang, X. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. USA 116, 2672–2680 (2019).
Conrad, M. & Proneth, B. Broken hearts: Iron overload, ferroptosis and cardiomyopathy. Cell Res. 29, 263–264 (2019).
Tang, L. J. et al. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic. Biol. Med. 162, 339–352 (2021).
Tang, L. J. et al. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn-Schmiedeberg’s Arch. Pharmacol. 394, 401–410 (2021).
Baba, Y. et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 314, H659–H668 (2018).
Chen, H. Y. et al. ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol. Med. 27, 14 (2021).
Li, W., Li, W., Wang, Y., Leng, Y. & Xia, Z. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 7, 267 (2021).
Tian, H. et al. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 27, 149–164 (2021).
Machado, S. E. et al. Counteraction of myocardial ferritin heavy chain deficiency by heme oxygenase-1. Int. J. Mol. Sci. 23, 8300 (2022).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 59, 298–308 (2015).
Feng, Y., Madungwe, N. B., Imam Aliagan, A. D., Tombo, N. & Bopassa, J. C. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem. Biophys. Res. Commun. 520, 606–611 (2019).
Zhang, T. et al. Mechanisms of ferroptosis regulating oxidative stress and energy metabolism in myocardial ischemia-reperfusion injury and a novel perspective of natural plant active ingrediets for its treatment. Biomed. Pharmacother. 165, 114706 (2023).
Zhao, K. et al. Broadening horizons: The role of ferroptosis in myocardial ischemia-reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 396, 2269–2286 (2023).
Ju, J. et al. Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J. Biomed. Sci. 30, 45 (2023).
Wu, T. et al. Circulating small extracellular vesicle-encapsulated SEMA5A-IT1 attenuates myocardial ischemia-reperfusion injury after cardiac surgery with cardiopulmonary bypass. Cell. Mol. Biol. Lett. 27, 95 (2022).
Lei, D., Li, B., Isa, Z., Ma, X. & Zhang, B. Hypoxia-elicited cardiac microvascular endothelial cell-derived exosomal miR-210-3p alleviate hypoxia/reoxygenation-induced myocardial cell injury through inhibiting transferrin receptor 1-mediated ferroptosis. Tissue Cell 79, 101956 (2022).
Zhou, X. et al. miR-190a-5p regulates cardiomyocytes response to ferroptosis via directly targeting GLS2. Biochem. Biophys. Res. Commun. 566, 9–15 (2021).
Zhang, G. Y., Gao, Y., Guo, X. Y., Wang, G. H. & Guo, C. X. MiR-199a-5p promotes ferroptosis-induced cardiomyocyte death responding to oxygen-glucose deprivation/reperfusion injury via inhibiting Akt/eNOS signaling pathway. Kaohsiung J. Med. Sci. 38, 1093–1102 (2022).
Liu, F. et al. Inhibition of miR-214-3p attenuates ferroptosis in myocardial infarction via regulating ME2. Biochem. Biophys. Res. Commun. 661, 64–ME74 (2023).
Fan, K. et al. The Egr-1/miR-15a-5p/GPX4 axis regulates ferroptosis in acute myocardial infarction. Eur. J. Pharmacol. 909, 174403 (2021).
Zhang, Z. et al. Frataxin inhibits the sensitivity of the myocardium to ferroptosis by regulating iron homeostasis. Free Radic. Biol. Med. 205, 305–317 (2023).
Ma, S. et al. USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front Physiol. 11, 551318 (2020).
Jiang, Y., Qiao, Y., He, D., Tian, A. & Li, Z. Adaptor protein HIP-55-mediated signalosome protects against ferroptosis in myocardial infarction. Cell Death Differ. 30, 825–838 (2023).
Herrmann, J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 17, 474–502 (2020).
Liu, Y. et al. Acyl-CoA thioesterase 1 prevents cardiomyocytes from Doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis. 11, 756 (2020).
Tadokoro, T. et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 5, e132747 (2020).
Zhang, H., Wang, Z., Liu, Z., Du, K. & Lu, X. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front. Cardiovasc. Med. 8, 685434 (2021).
Zhuang, S. et al. METTL14 promotes doxorubicin-induced cardiomyocyte ferroptosis by regulating the KCNQ1OT1-miR-7-5p-TFRC axis. Cell Biol. Toxicol. 39, 1015–1035 (2023).
Wang, Y. et al. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 29, 1982–1995 (2022).
Liao, H. H. et al. Activation of AMPKα2 attenuated doxorubicin-induced cardiotoxicity via inhibiting lipid peroxidation associated ferroptosis. Free Radic. Biol. Med. 205, 275–290 (2023).
Hou, K. et al. Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine 69, 103456 (2021).
Kitakata, H. et al. MITOL/MARCH5 determines the susceptibility of cardiomyocytes to doxorubicin-induced ferroptosis by regulating GSH. Homeost. J. Mol. Cell. Cardiol. 161, 116–129 (2021).
Zhou, Y. J. et al. The SPATA2/CYLD pathway contributes to doxorubicin-induced cardiomyocyte ferroptosis via enhancing ferritinophagy. Chem. Biol. Interact. 368, 110205 (2022).
Su, H., Cantrell, A. C., Chen, J. X., Gu, W. & Zeng, H. SIRT3 deficiency enhances ferroptosis and promotes cardiac fibrosis via p53 acetylation. Cells. 12, 1428 (2023).
Zhuang, Y. et al. MiR-375-3p Promotes Cardiac Fibrosis by Regulating the Ferroptosis Mediated by GPX4. Comput. Intell. Neurosci. 2022, 9629158 (2022).
Zheng, H., Shi, L., Tong, C., Liu, Y. & Hou, M. circSnx12 is involved in ferroptosis during heart failure by targeting miR-224-5p. Front. Cardiovasc. Med. 8, 656093 (2021).
Li, S. et al. PGAM5 expression levels in heart failure and protection ROS-induced oxidative stress and ferroptosis by Keap1/Nrf2. Clin. Exp. Hypertens. 45, 2162537 (2023).
Zhu, M. et al. STAT3 signaling promotes cardiac injury by upregulating NCOA4-mediated ferritinophagy and ferroptosis in high-fat-diet fed mice. Free Radic. Biol. Med. 201, 111–125 (2023).
Zhong, F. Y. et al. The role of CD147 in pathological cardiac hypertrophy is regulated by glycosylation. Oxid. Med. Cell Longev. 2022, 6603296 (2022).
Li, N. et al. Targeting ferroptosis as a novel approach to alleviate aortic dissection. Int. J. Biol. Sci. 18, 4118–4134 (2022).
Nabzdyk, C. S., Couture, E. J., Shelton, K., Cudemus, G. & Bittner, E. A. Sepsis induced cardiomyopathy: Pathophysiology and use of mechanical circulatory support for refractory shock. J. Crit. Care 54, 228–234 (2019).
Hollenberg, S. M. & Singer, M. Pathophysiology of sepsis-induced cardiomyopathy. Nat. Rev. Cardiol. 18, 424–434 (2021).
Chen, Z. et al. TMEM43 protects against sepsis-induced cardiac injury via inhibiting ferroptosis in mice. Cells. 11, 2992 (2022).
Wang, C. et al. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol. Med. Rep. 22, 175–184 (2020).
Li, N. et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 160, 303–318 (2020).
Kong, C. et al. ICA69 aggravates ferroptosis causing septic cardiac dysfunction via STING trafficking. Cell Death Discov. 8, 187 (2022).
Huang, Y. et al. Lipocalin-2 in neutrophils induces ferroptosis in septic cardiac dysfunction via increasing labile iron pool of cardiomyocytes. Front. Cardiovasc. Med. 9, 922534 (2022).
Xiang, X., Gao, J., Su, D. & Shi, D. The advancements in targets for ferroptosis in liver diseases. Front. Med. 10, 1084479 (2023).
Lu, Y. et al. Ferroptosis as an emerging therapeutic target in liver diseases. Front. Pharm. 14, 1196287 (2023).
Huang, X. et al. The emerging roles of ferroptosis in organ fibrosis and its potential therapeutic effect. Int. Immunopharmacol. 116, 109812 (2023).
Li, L. & Zhu, Z. Pharmacological modulation of ferroptosis as a therapeutic target for liver fibrosis. Front. Pharm. 13, 1071844 (2022).
Pan, Y. et al. Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants 11, 2196 (2022).
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Li, J. et al. Role of liensinine in sensitivity of activated macrophages to ferroptosis and in acute liver injury. Cell Death Discov. 9, 189 (2023).
Dai, W. et al. Liver protection of a low-polarity fraction from ficus pandurata hance, prepared by supercritical CO(2) fluid extraction, on CCl(4)-induced acute liver injury in mice via inhibiting apoptosis and ferroptosis mediated by strengthened antioxidation. Molecules 28, 2078 (2023).
He, X. et al. Mitoquinone protects against acetaminophen-induced liver injury in an FSP1-dependent and GPX4-independent manner. Toxicol. Appl. Pharmacol. 465, 116452 (2023).
Wei, Y. Y. et al. Acute liver injury induced by carbon tetrachloride reversal by Gandankang aqueous extracts through nuclear factor erythroid 2-related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 251, 114527 (2023).
Li, Y. et al. Ginsenoside Rd inhibited ferroptosis to alleviate CCl(4)-induced acute liver injury in mice via cGAS/STING pathway. Am. J. Chin. Med. 51, 91–105 (2023).
Liu, J. et al. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol. Res. 187, 106563 (2023).
Zhao, T. et al. Regulating Nrf2-GPx4 axis by bicyclol can prevent ferroptosis in carbon tetrachloride-induced acute liver injury in mice. Cell Death Discov. 8, 380 (2022).
Tak, J. et al. Gα12 overexpression in hepatocytes by ER stress exacerbates acute liver injury via ROCK1-mediated miR-15a and ALOX12 dysregulation. Theranostics 12, 1570–1588 (2022).
Wang, C. et al. Ulinastatin protects against acetaminophen-induced liver injury by alleviating ferroptosis via the SIRT1/NRF2/HO-1 pathway. Am. J. Transl. Res. 13, 6031–6042 (2021).
Lin, F. et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 13, 271 (2022).
Wu, Y. et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 29, 1705–1718 (2022).
Friedman, S. L. & Pinzani, M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology 75, 473–488 (2022).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Kim, K. M., Cho, S. S. & Ki, S. H. Emerging roles of ferroptosis in liver pathophysiology. Arch. Pharm. Res. 43, 985–996 (2020).
Pan, Q., Luo, Y., Xia, Q. & He, K. Ferroptosis and liver fibrosis. Int J. Med. Sci. 18, 3361–3366 (2021).
Wu, J. et al. Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov. 7, 276 (2021).
Chen, J., Li, X., Ge, C., Min, J. & Wang, F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 29, 467–480 (2022).
Zhou, X. et al. Ferroptosis in chronic liver diseases: opportunities and challenges. Front. Mol. Biosci. 9, 928321 (2022).
Zhao, W. et al. Yes-associated protein inhibition ameliorates liver fibrosis and acute and chronic liver failure by decreasing ferroptosis and necroptosis. Heliyon 9, e15075 (2023).
Xu, L. et al. Doxofylline ameliorates liver fibrosis by regulating the ferroptosis signaling pathway. Front. Pharm. 14, 1135366 (2023).
Zhang, X. et al. Targeting hepatic stellate cell death to reverse hepatic fibrosis. Curr. Drug Targets 24, 568–583 (2023).
Du, K. et al. Targeting YAP-mediated HSC death susceptibility and senescence for treatment of liver fibrosis. Hepatology 77, 1998–2015 (2023).
Zhang, Z. et al. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy 16, 1482–1505 (2020).
Zhang, Z. et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy 14, 2083–2103 (2018).
Zhang, Z. et al. The BRD7-P53-SLC25A28 axis regulates ferroptosis in hepatic stellate cells. Redox Biol. 36, 101619 (2020).
Zhu, Y. et al. TRIM26 induces ferroptosis to inhibit hepatic stellate cell activation and mitigate liver fibrosis through mediating SLC7A11 ubiquitination. Front. Cell Dev. Biol. 9, 644901 (2021).
Lv, T. et al. Mitochondrial general control of amino acid synthesis 5 like 1 promotes nonalcoholic steatohepatitis development through ferroptosis-induced formation of neutrophil extracellular traps. Clin. Transl. Med. 13, e1325 (2023).
Guo, J. et al. Hepatocyte-specific TMEM16A deficiency alleviates hepatic ischemia/reperfusion injury via suppressing GPX4-mediated ferroptosis. Cell Death Dis. 13, 1072 (2022).
Xu, Y., Li, Y., Li, J. & Chen, W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 53, 102349 (2022).
Liu, J., Yang, G. & Zhang, H. Glyphosate-triggered hepatocyte ferroptosis via suppressing Nrf2/GSH/GPX4 axis exacerbates hepatotoxicity. Sci. Total Environ. 862, 160839 (2023).
Meyer, N. J., Gattinoni, L. & Calfee, C. S. Acute respiratory distress syndrome. Lancet 398, 622–637 (2021).
Matthay, M. A. et al. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 5, 18 (2019).
Liu, X., Zhang, J. & Xie, W. The role of ferroptosis in acute lung injury. Mol. Cell. Biochem. 477, 1453–1461 (2022).
Li, M. D. et al. Arsenic induces ferroptosis and acute lung injury through mtROS-mediated mitochondria-associated endoplasmic reticulum membrane dysfunction. Ecotoxicol. Environ. Saf. 238, 113595 (2022).
Qiang, Z. et al. Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A11. Oxid. Med. Cell Longev. 2020, 5146982 (2020).
Yang, Y. et al. STAT6 inhibits ferroptosis and alleviates acute lung injury via regulating P53/SLC7A11 pathway. Cell Death Dis. 13, 530 (2022).
Chen, H. et al. SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int. J. Hyperth. 39, 977–986 (2022).
Zhang, H. et al. Neutrophil extracellular traps mediate m(6)A modification and regulates sepsis-associated acute lung injury by activating ferroptosis in alveolar epithelial cells. Int. J. Biol. Sci. 18, 3337–3357 (2022).
Wang, Y. M. et al. Mucin 1 inhibits ferroptosis and sensitizes vitamin E to alleviate sepsis-induced acute lung injury through GSK3β/Keap1-Nrf2-GPX4 pathway. Oxid. Med. Cell Longev. 2022, 2405943 (2022).
Wang, Y. et al. AUF1 protects against ferroptosis to alleviate sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell. Mol. Life Sci. 79, 228 (2022).
Xu, B., Wang, H. & Chen, Z. Puerarin inhibits ferroptosis and inflammation of lung injury caused by sepsis in LPS induced lung epithelial cells. Front. Pediatr. 9, 706327 (2021).
Shimizu, J., Murao, A., Nofi, C., Wang, P. & Aziz, M. Extracellular CIRP promotes GPX4-mediated ferroptosis in sepsis. Front. Immunol. 13, 903859 (2022).
Zhang, J. et al. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front. Immunol. 13, 884362 (2022).
Wang, W., Xu, R., Zhao, H., Xiong, Y. & He, P. CircEXOC5 promotes ferroptosis by enhancing ACSL4 mRNA stability via binding to PTBP1 in sepsis-induced acute lung injury. Immunobiology 227, 152219 (2022).
Shen, K. et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 62, 102655 (2023).
Günes Günsel, G. et al. The arginine methyltransferase PRMT7 promotes extravasation of monocytes resulting in tissue injury in COPD. Nat. Commun. 13, 1303 (2022).
Zhang, Z. et al. Hypermethylation of the Nrf2 promoter induces ferroptosis by inhibiting the Nrf2-GPX4 Axis in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 16, 3347–3362 (2021).
Cui, Y. et al. MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells. Cell Death Dis. 14, 2 (2023).
Yang, M. et al. Mitochondrial translocation of P66Shc aggravates cisplatin-induced AKI by promoting ferroptosis. Curr. Med. Chem. 30, 744–756 (2023).
Zhou, J. et al. MicroRNA-214-3p aggravates ferroptosis by targeting GPX4 in cisplatin-induced acute kidney injury. Cell Stress Chaperones 27, 325–336 (2022).
Jin, J., Wang, Y., Zheng, D., Liang, M. & He, Q. A novel identified circular RNA, mmu_mmu_circRNA_0000309, involves in germacrone-mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting miR-188-3p/GPX4 signaling axis. Antioxid. Redox Signal. 36, 740–759 (2022).
Ye, Z. et al. p53 deacetylation alleviates calcium oxalate deposition-induced renal fibrosis by inhibiting ferroptosis. Biomed. Pharmacother. 164, 114925 (2023).
Shi, L. et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am. J. Transplant. 23, 11–25 (2023).
Ding, C. et al. miR-182-5p and miR-378a-3p regulate ferroptosis in I/R-induced renal injury. Cell Death Dis. 11, 929 (2020).
Pan, J. et al. Inhibition of USP14 suppresses ROS-dependent ferroptosis and alleviates renal ischemia/reperfusion injury. Cell Biochem. Biophys. 81, 87–96 (2023).
Sun, X. et al. TRIM21 ubiquitylates GPX4 and promotes ferroptosis to aggravate ischemia/reperfusion-induced acute kidney injury. Life Sci. 321, 121608 (2023).
Dong, B. et al. USP7 accelerates FMR1-mediated ferroptosis by facilitating TBK1 ubiquitination and DNMT1 deubiquitination after renal ischemia-reperfusion injury. Inflamm. Res. 71, 1519–1533 (2022).
Zhou, Z. & Zhang, H. CHAC1 exacerbates LPS-induced ferroptosis and apoptosis in HK-2 cells by promoting oxidative stress. Allergol. Immunopathol. 51, 99–110 (2023).
Fujiki, K., Inamura, H., Sugaya, T. & Matsuoka, M. Blockade of ALK4/5 signaling suppresses cadmium- and erastin-induced cell death in renal proximal tubular epithelial cells via distinct signaling mechanisms. Cell Death Differ. 26, 2371–2385 (2019).
Tan, Q. L. et al. TIGAR protects against adenine-induced ferroptosis in human proximal tubular epithelial cells by activating the mTOR/S6KP70 axis. Nutr. Cancer 75, 1464–1472 (2023).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
Scholz, H. et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat. Rev. Nephrol. 17, 335–349 (2021).
Shi, Y. et al. Ferroptosis: a new mechanism of traditional Chinese medicine compounds for treating acute kidney injury. Biomed. Pharmacother. 163, 114849 (2023).
Bayır, H., Dixon, S. J., Tyurina, Y. Y., Kellum, J. A. & Kagan, V. E. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat. Rev. Nephrol. 19, 315–336 (2023).
Zhou, Y. et al. The role of ferroptosis in the development of acute and chronic kidney diseases. J. Cell. Physiol. 237, 4412–4427 (2022).
DeFronzo, R. A., Reeves, W. B. & Awad, A. S. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17, 319–334 (2021).
Kato, M. & Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 15, 327–345 (2019).
Wang, H. et al. Emerging role of ferroptosis in diabetic kidney disease: molecular mechanisms and therapeutic opportunities. Int. J. Biol. Sci. 19, 2678–2694 (2023).
Wang, Y., Chang, D., Zhao, M. & Chen, M. Dapagliflozin alleviates diabetic kidney disease via HIF1α/HO1 mediated ferroptosis. Antioxid. Redox Signal. https://doi.org/10.1089/ars.2022.0169 (2023).
Chen, J. et al. Inhibition of fatty acid β-oxidation by fatty acid binding protein 4 induces ferroptosis in HK2 cells under high glucose conditions. Endocrinol. Metab. 38, 226–244 (2023).
Lu, Q. et al. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med. 195, 89–102 (2023).
Wu, Z., Li, D., Tian, D., Liu, X. & Wu, Z. Aspirin mediates protection from diabetic kidney disease by inducing ferroptosis inhibition. PLoS One 17, e0279010 (2022).
Wang, M. Z. et al. Inhibition of ferroptosis of renal tubular cells with total flavones of Abelmoschus manihot alleviates diabetic tubulopathy. Anatom. Record. 306, 3199–3213 (2023).
Li, L., Fu, H. & Liu, Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat. Rev. Nephrol. 18, 545–557 (2022).
Zhou, J., Tan, Y., Wang, R. & Li, X. Role of ferroptosis in fibrotic diseases. J. Inflamm. Res. 15, 3689–3708 (2022).
Zhang, H. Y., Cheng, M., Zhang, L. & Wang, Y. P. Ferroptosis and renal fibrosis: a new target for the future (Review). Exp. Ther. Med. 25, 13 (2023).
Yang, S. et al. Salidroside alleviates renal fibrosis in SAMP8 mice by inhibiting ferroptosis. Molecules 27, 8039 (2022).
Zhang, Y. et al. Therapeutic implications of ferroptosis in renal fibrosis. Front. Mol. Biosci. 9, 890766 (2022).
Wang, J. et al. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 8, 127 (2022).
Yang, L. et al. Tocilizumab mimotope alleviates kidney injury and fibrosis by inhibiting IL-6 signaling and ferroptosis in UUO model. Life Sci. 261, 118487 (2020).
Huang, P. et al. Crosstalk between gut microbiota and renal ischemia/reperfusion injury. Front. Cell Infect. Microbiol. 12, 1015825 (2022).
Qi, Y. et al. Mitoglitazone ameliorates renal ischemia/reperfusion injury by inhibiting ferroptosis via targeting mitoNEET. Toxicol. Appl. Pharmacol. 465, 116440 (2023).
Zhao, Z. et al. Cytoplasmic HMGB1 induces renal tubular ferroptosis after ischemia/reperfusion. Int. Immunopharmacol. 116, 109757 (2023).
Tao, W. H. et al. Dexmedetomidine attenuates ferroptosis-mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via α2-AR. Front. Pharm. 13, 782466 (2022).
Tao, W. et al. miR-3587 inhibitor attenuates ferroptosis following renal ischemia-reperfusion through HO-1. Front Mol. Biosci. 8, 789927 (2021).
Zhao, Z. et al. XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis. 11, 629 (2020).
Su, L. et al. Pannexin 1 mediates ferroptosis that contributes to renal ischemia/reperfusion injury. J. Biol. Chem. 294, 19395–19404 (2019).
Zarbock, A. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 19, 401–417 (2023).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ. 364, k4891 (2019).
Manrique-Caballero, C. L., Del Rio-Pertuz, G. & Gomez, H. Sepsis-associated acute kidney injury. Crit. Care Clin. 37, 279–301 (2021).
Alobaidi, R., Basu, R. K., Goldstein, S. L. & Bagshaw, S. M. Sepsis-associated acute kidney injury. Semin. Nephrol. 35, 2–11 (2015).
Xiao, J. et al. Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Biosci. 11, 221 (2021).
Liang, N. N. et al. Mitochondria-derived reactive oxygen species are involved in renal cell ferroptosis during lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 107, 108687 (2022).
Yao, W. et al. Inhibition of the NADPH oxidase pathway reduces ferroptosis during septic renal injury in diabetic mice. Oxid. Med. Cell Longev. 2022, 1193734 (2022).
Zhang, H. et al. The regulation of LPCAT3 by miR-124-3p.1 in acute kidney injury suppresses cell proliferation by disrupting phospholipid metabolism. Biochem. Biophys. Res. Commun. 604, 37–42 (2022).
Babar, Q. et al. Novel epigenetic therapeutic strategies and targets in cancer. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166552 (2022).
Yang, C. et al. Tiliroside targets TBK1 to induce ferroptosis and sensitize hepatocellular carcinoma to sorafenib. Phytomedicine 111, 154668 (2023).
Peng, Y. et al. Corosolic acid sensitizes ferroptosis by upregulating HERPUD1 in liver cancer cells. Cell Death Discov. 8, 376 (2022).
Su, Y. et al. Dihydroartemisinin induces ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid. Med. Cell Longev. 2021, 3456725 (2021).
Huang, Q. et al. High-throughput screening identification of a small-molecule compound that induces ferroptosis and attenuates the invasion and migration of hepatocellular carcinoma cells by targeting the STAT3/GPX4 axis. Int. J. Oncol. 62, 42 (2023).
Chen, W. et al. Modulation of the p38 MAPK pathway by anisomycin promotes ferroptosis of hepatocellular carcinoma through phosphorylation of H3S10. Oxid. Med. Cell Longev. 2022, 6986445 (2022).
Zhang, W., Jiang, B., Liu, Y., Xu, L. & Wan, M. Bufotalin induces ferroptosis in non-small cell lung cancer cells by facilitating the ubiquitination and degradation of GPX4. Free Radic. Biol. Med. 180, 75–84 (2022).
Liu, Y. et al. Pharmacological inhibition of sphingolipid synthesis reduces ferroptosis by stimulating the HIF-1 pathway. iScience 25, 104533 (2022).
Chan, L. S. et al. Selenite as a dual apoptotic and ferroptotic agent synergizes with EGFR and KRAS inhibitors with epigenetic interference. Clin. Epigenet. 15, 36 (2023).
Wei, Y. et al. Eupaformosanin induces apoptosis and ferroptosis through ubiquitination of mutant p53 in triple-negative breast cancer. Eur. J. Pharmacol. 924, 174970 (2022).
Ding, Y. et al. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 14, 19 (2021).
Li, G. et al. A PARP1 PROTAC as a novel strategy against PARP inhibitor resistance via promotion of ferroptosis in p53-positive breast cancer. Biochem. Pharmacol. 206, 115329 (2022).
Li, H. et al. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Biochem. Biophys. Res. Commun. 585, 111–116 (2021).
Chen, T. C. et al. AR ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting GPX4-Mediated redox homeostasis. Redox Biol. 30, 101413 (2020).
Nie, X. H. et al. Paeoniflorin regulates NEDD4L/STAT3 pathway to induce ferroptosis in human glioma cells. J. Oncol. 2022, 6093216 (2022).
Zhou, Y., Qian, W., Li, X. & Wei, W. NF-κB inhibitor myrislignan induces ferroptosis of glioblastoma cells via regulating epithelial-mesenchymal transformation in a slug-dependent manner. Oxid. Med. Cell Longev. 2023, 7098313 (2023).
Hu, S. et al. A novel redox modulator induces a GPX4-mediated cell death that is dependent on iron and reactive oxygen species. J. Med. Chem. 63, 9838–9855 (2020).
Guo, Z. & Zhang, Y. Allicin promotes autophagy and ferroptosis in esophageal squamous cell carcinoma by activating AMPK/mTOR signaling. Heliyon 8, e11005 (2022).
Wang, X. et al. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J. Biochem. Mol. Toxicol. 37, e23368 (2023).
Sekhar, K. R. et al. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroidcancer. Sci. Rep. 12, 19396 (2022).
Zhang, L., Li, D. C. & Liu, L. F. Paeonol: pharmacological effects and mechanisms of action. Int. Immunopharmacol. 72, 413–421 (2019).
Ramazi, S., Fahanik-Babaei, J., Mohamadi-Zarch, S. M., Baluchnejadmojarad, T. & Roghani, M. Paeonol exerts neuroprotective and anticonvulsant effects in intrahippocampal kainate model of temporal lobe epilepsy. J. Chem. Neuroanat. 124, 102121 (2022).
Liu, D. H., Agbo, E., Zhang, S. H. & Zhu, J. L. Anticonvulsant and neuroprotective effects of paeonol in epileptic rats. Neurochem. Res. 44, 2556–2565 (2019).
Latif, S. et al. Antioxidant and neuroprotective effects of paeonol against oxidative stress and altered carrier-mediated transport system on NSC-34 Cell Lines. Antioxidants 11, 1392 (2022).
Liao, W. Y. et al. Neuroprotective effect of paeonol mediates anti-inflammation via suppressing toll-like receptor 2 and toll-like receptor 4 signaling pathways in cerebral ischemia-reperfusion injured rats. Evid. Based Complement. Altern. Med. 2016, 3704647 (2016).
Adki, K. M. & Kulkarni, Y. A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 250, 117544 (2020).
Pourmohammadi, S., Roghani, M., Kiasalari, Z. & Khalili, M. Paeonol ameliorates cuprizone-induced hippocampal demyelination and cognitive deficits through inhibition of oxidative and inflammatory events. J. Mol. Neurosci. 72, 748–758 (2022).
Adki, K. M. & Kulkarni, Y. A. Neuroprotective effect of paeonol in streptozotocin-induced diabetes in rats. Life Sci. 271, 119202 (2021).
Zhao, H., Wang, X., Liu, S. & Zhang, Q. Paeonol regulates NLRP3 inflammasomes and pyroptosis to alleviate spinal cord injury of rat. BMC Neurosci. 23, 16 (2022).
Tseng, Y. T., Hsu, Y. Y., Shih, Y. T. & Lo, Y. C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock 37, 312–318 (2012).
Nam, K. N. et al. Paeonol attenuates inflammation-mediated neurotoxicity and microglial activation. Neural Regen. Res. 8, 1637–1643 (2013).
Meng, S., Wang, B. & Li, W. Paeonol administration alleviates cognitive deficits and attenuates neural pathological changes in APP/PS1 mice. J. Integr. Neurosci. 20, 1001–1010 (2021).
Liu, J. et al. Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neurosci. Lett. 549, 63–68 (2013).
Ghalami, J., Baluchnejad Mojarad, T., Mansouri, M., Khamse, S. & Roghani, M. Paeonol protection against intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease. Basic Clin. Neurosci. 12, 43–56 (2021).
Yang, C. T. et al. Paeonol promotes hippocampal synaptic transmission: the role of the Kv2.1 potassium channel. Eur. J. Pharmacol. 827, 227–237 (2018).
Zhang, H. et al. Paeonol at certain doses alleviates aggressive and anxiety-like behaviours in two premenstrual dysphoric disorder rat models. Front. Psychiatry 11, 295 (2020).
Jin, H. et al. Paeonol attenuates isoflurane anesthesia-induced hippocampal neurotoxicity via modulation of JNK/ERK/P38MAPK pathway and regulates histone acetylation in neonatal rat. J. Matern. Fetal Neonatal Med. 33, 81–91 (2020).
Gyawali, A., Krol, S. & Kang, Y. S. Involvement of a novel organic cation transporter in paeonol transport across the blood-brain barrier. Biomol. Ther. 27, 290–301 (2019).
Tayanloo-Beik, A., Kiasalari, Z. & Roghani, M. Paeonol ameliorates cognitive deficits in streptozotocin murine model of sporadic Alzheimer’s disease via attenuation of oxidative stress, inflammation, and mitochondrial dysfunction. J. Mol. Neurosci. 72, 336–348 (2022).
Wang, X. et al. Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med. 77, 1695–1701 (2011).
Qiu, C. et al. Paeonol ameliorates CFA-induced inflammatory pain by inhibiting HMGB1/TLR4/NF-κB p65 pathway. Metab. Brain Dis. 36, 273–283 (2021).
Sun, Y. et al. Primary studies on construction and evaluation of ion-sensitive in situ gel loaded with paeonol-solid lipid nanoparticles for intranasal drug delivery. Int. J. Nanomed. 15, 3137–3160 (2020).
Chen, F., Qi, W., Sun, J., Simpkins, J. W. & Yuan, D. Urinary metabolites of isorhynchophylline in rats and their neuroprotective activities in the HT22 cell assay. Fitoterapia 97, 156–163 (2014).
Zhou, J. Y. & Zhou, S. W. Isorhynchophylline: a plant alkaloid with therapeutic potential for cardiovascular and central nervous system diseases. Fitoterapia 83, 617–626 (2012).
Li, H. Q., Ip, S. P., Zheng, G. Q., Xian, Y. F. & Lin, Z. X. Isorhynchophylline alleviates learning and memory impairments induced by aluminum chloride in mice. Chin. Med. 13, 29 (2018).
Deng, Y. et al. Isorhynchophylline ameliorates cerebral ischemia/reperfusion injury by inhibiting CX3CR1-mediated microglial activation and neuroinflammation. Front. Pharm. 12, 574793 (2021).
Li, Q., Niu, C., Zhang, X. & Dong, M. Gastrodin and isorhynchophylline synergistically inhibit MPP(+)-induced oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3β pathways: involvement of Nrf2 nuclear translocation. ACS Chem. Neurosci. 9, 482–493 (2018).
Xian, Y. F. et al. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 32, 353–360 (2012).
Xian, Y. F., Fan, D., Ip, S. P., Mao, Q. Q. & Lin, Z. X. Antidepressant-like effect of isorhynchophylline in mice. Neurochem. Res. 42, 678–685 (2017).
Li, X. M., Zhang, X. J. & Dong, M. X. Isorhynchophylline attenuates MPP(+)-induced apoptosis through endoplasmic reticulum stress- and mitochondria-dependent pathways in PC12 cells: involvement of antioxidant activity. Neuromol. Med. 19, 480–492 (2017).
Xian, Y. F. et al. Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J. Alzheimer’s Dis. 39, 331–346 (2014).
Xian, Y. F. et al. Isorhynchophylline protects PC12 cells against beta-amyloid-induced apoptosis via PI3K/Akt signaling pathway. Evid. Based Complement. Altern. Med. 2013, 163057 (2013).
Wang, X., Wang, Z., Cao, J., Dong, Y. & Chen, Y. Melatonin alleviates acute sleep deprivation-induced memory loss in mice by suppressing hippocampal ferroptosis. Front Pharm. 12, 708645 (2021).
Dou, X. et al. Therapeutic potential of melatonin in the intervertebral disc degeneration through inhibiting the ferroptosis of nucleus pulpous cells. J. Cell. Mol. Med. 27, 2340–2353 (2023).
Ren, C. et al. Melatonin reduces radiation-induced ferroptosis in hippocampal neurons by activating the PKM2/NRF2/GPX4 signaling pathway. Prog. Neuropsychopharmacol. Biol. Psychiatry 126, 110777 (2023).
Zhang, F. et al. Melatonin alleviates retinal ischemia-reperfusion injury by inhibiting p53-mediated ferroptosis. Antioxidants 12, 1173 (2023).
Gao, Y. et al. Melatonin ameliorates neurological deficits through MT2/IL-33/ferritin H signaling-mediated inhibition of neuroinflammation and ferroptosis after traumatic brain injury. Free Radic. Biol. Med. 199, 97–112 (2023).
Wu, C. et al. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free Radic. Biol. Med. 178, 271–294 (2022).
Chen, Y. et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol. Res. 177, 106122 (2022).
Bian, J. et al. Celastrol confers ferroptosis resistance via AKT/GSK3β signaling in high-fat diet-induced cardiac injury. Free Radic. Biol. Med. 200, 36–46 (2023).
Yi, J. et al. Berberine alleviates liver fibrosis through inducing ferrous redox to activate ROS-mediated hepatic stellate cells ferroptosis. Cell Death Discov. 7, 374 (2021).
Wu, A. et al. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 46, 102131 (2021).
Li, Y. et al. Iron regulatory protein 2 is required for artemether -mediated anti-hepatic fibrosis through ferroptosis pathway. Free Radic. Biol. Med. 160, 845–859 (2020).
Xu, J. et al. Urolithin C reveals anti-NAFLD potential via AMPK-ferroptosis axis and modulating gut microbiota. Naunyn-Schmiedeberg’s Arch. Pharm. 396, 2687–2699 (2023).
Zhang, Q. Q. et al. AGK2 pre-treatment protects against thioacetamide-induced acute liver failure via regulating the MFN2-PERK axis and ferroptosis signaling pathway. HBPD Int. 16, S1499-3872(23)00037-1 (2023).
Li, J. et al. Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses. Cell. Mol. Biol. Lett. 27, 29 (2022).
Li, N., Xiong, R., Li, G., Wang, B. & Geng, Q. PM2.5 contributed to pulmonary epithelial senescence and ferroptosis by regulating USP3-SIRT3-P53 axis. Free Radic. Biol. Med. 205, 291–304 (2023).
Li, X. et al. Pretreatment with roxadustat (FG-4592) attenuates folic acid-induced kidney injury through antiferroptosis via Akt/GSK-3β/Nrf2 pathway. Oxid. Med. Cell Longev. 2020, 6286984 (2020).
Yu, M. et al. Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chem. Biol. Interact. 382, 110607 (2023).
Xu, Z. et al. Dihydromyricetin attenuates cisplatin-induced acute kidney injury by reducing oxidative stress, inflammation and ferroptosis. Toxicol. Appl. Pharmacol. 473, 116595 (2023).
Qi, H. et al. Myo-inositol supplementation alleviates cisplatin-induced acute kidney injury via inhibition of ferroptosis. Cells. 12, 16 (2022).
Huang, B., Wen, W. & Ye, S. Dapagliflozin ameliorates renal tubular ferroptosis in diabetes via SLC40A1 stabilization. Oxid. Med Cell Longev. 2022, 9735555 (2022).
Chen, J. et al. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 156, 113953 (2022).
Wang, X. et al. Schisandrin A from Schisandra chinensis attenuates ferroptosis and NLRP3 inflammasome-mediated pyroptosis in diabetic nephropathy through mitochondrial damage by AdipoR1 ubiquitination. Oxid. Med. Cell Longev. 2022, 5411462 (2022).
Qin, L. Y. et al. Therapeutic potential of astragaloside IV against adriamycin-induced renal damage in rats via ferroptosis. Front. Pharm. 13, 812594 (2022).
Paniri, A., Hosseini, M. M. & Akhavan-Niaki, H. Alzheimer’s disease-related epigenetic changes: novel therapeutic targets. Mol. Neurobiol. (2023).
Yuan, M. et al. Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduct. Target Ther. 8, 309 (2023).
Micale, V. et al. Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol. Ther. 241, 108279 (2023).
Morris-Blanco, K. C. et al. Epigenetic mechanisms and potential therapeutic targets in stroke. J. Cereb. Blood Flow. Metab. 42, 2000–2016 (2022).
Liu, Z. Y. et al. Crosstalk between oxidative stress and epigenetic marks: new roles and therapeutic implications in cardiac fibrosis. Redox Biol. 65, 102820 (2023).
Sum, H. & Brewer, A. C. Epigenetic modifications as therapeutic targets in atherosclerosis: a focus on DNA methylation and non-coding RNAs. Front. Cardiovasc. Med. 10, 1183181 (2023).
Lin, L. C. et al. Mitochondrial quality control in cardiac fibrosis: epigenetic mechanisms and therapeutic strategies. Metab. Clin. Exp. 145, 155626 (2023).
Kraus, L. Targeting epigenetic regulation of cardiomyocytes through development for therapeutic cardiac regeneration after heart failure. Int. J. Mol. Sci. 23, 11878 (2022).
Li, D., Nie, J., Han, Y. & Ni, L. Epigenetic mechanism and therapeutic implications of atrial fibrillation. Front. Cardiovasc. Med. 8, 763824 (2021).
Theys, C., Lauwers, D., Perez-Novo, C. & Vanden Berghe, W. PPARα in the epigenetic driver seat of NAFLD: new therapeutic opportunities for epigenetic drugs. Biomedicines. 10, 3041 (2022).
Karimi, M., Abiri, B., Guest, P. C. & Vafa, M. Therapeutic effects of resveratrol on nonalcoholic fatty liver disease through inflammatory, oxidative stress, metabolic, and epigenetic modifications. Methods Mol. Biol. 2343, 19–35 (2022).
Ho, L., Hossen, N., Nguyen, T., Vo, A. & Ahsan, F. Epigenetic mechanisms as emerging therapeutic targets and microfluidic chips application in pulmonary arterial hypertension. Biomedicines 10, 170 (2022).
Avci, E., Sarvari, P., Savai, R., Seeger, W. & Pullamsetti, S. S. Epigenetic mechanisms in parenchymal lung diseases: bystanders or therapeutic targets. Int. J. Mol. Sci. 23, 546 (2022).
Cherneva, R. V. & Kostadinov, D. Epigenetic targets for therapeutic approaches in COPD and asthma. Nutrigenomics – possible or illusive. Folia Med. 61, 358–369 (2019).
Martinez-Moreno, J. M. et al. Epigenetic modifiers as potential therapeutic targets in diabetic kidney disease. Int. J. Mol. Sci. 21, 4113 (2020).
Guo, C., Dong, G., Liang, X. & Dong, Z. Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat. Rev. Nephrol. 15, 220–239 (2019).
Hayashi, K. & Itoh, H. Transcription factors and epigenetic modulation: its therapeutic implication in chronic kidney disease. Arch. Immunol. Ther. Exp. 63, 193–196 (2015).
Morel, D., Almouzni, G., Soria, J. C. & Postel-Vinay, S. Targeting chromatin defects in selected solid tumors based on oncogene addiction, synthetic lethality and epigenetic antagonism. Ann. Oncol. 28, 254–269 (2017).
Pfister, S. X. & Ashworth, A. Marked for death: targeting epigenetic changes in cancer. Nat. Rev. Drug Discov. 16, 241–263 (2017).
Morel, D., Jeffery, D., Aspeslagh, S., Almouzni, G. & Postel-Vinay, S. Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise. Nat. Rev. Clin. Oncol. 17, 91–107 (2020).
Topper, M. J., Vaz, M., Marrone, K. A., Brahmer, J. R. & Baylin, S. B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 75–90 (2020).
Zhang, Y., Koppula, P. & Gan, B. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle 18, 773–783 (2019).
Sun, J. et al. Midbrain dopamine oxidation links ubiquitination of glutathione peroxidase 4 to ferroptosis of dopaminergic neurons. J. Clin. Investig. 133, e173110 (2023).
Liu, Y. et al. UHRF1-mediated ferroptosis promotes pulmonary fibrosis via epigenetic repression of GPX4 and FSP1 genes. Cell Death Dis. 13, 1070 (2022).
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (82271895, 82072752, and 81172498), the Science Foundation of AMHT (2020YK02, 2021YK05, 2022YK01), the Science Foundation of CASIC (2020-LCYL-009), the Science Foundation of ASCH (YN202104, YN202305), the Hygiene and Health Development Scientific Research Fostering Plan of Haidian District Beijing (HP2021-19-50701), and Key Programs of Science Foundation of Heilongjiang Province (ZD2019H009).
Author information
Authors and Affiliations
Contributions
Y.W., S.W. and H.W. researched data for the article and contributed substantially to discussion of the content. Y.W., S.W. and H.W. wrote the article. Y.L., Y.X. and W.Z reviewed the manuscript. J.W., J.C. and H.W reviewed and/or edited the manuscript before submission. Y.W., J.S.F. and H.W. prepared figures. S.W. and Y.X. modified the manuscript contents. Y.W. and H.W. conceived of and designed the study. H.W., J.W. and Y.F. provided administrative support. All authors analyzed and interpreted the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Hu, J., Wu, S. et al. Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases. Sig Transduct Target Ther 8, 449 (2023). https://doi.org/10.1038/s41392-023-01720-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01720-0