Abstract
Psoriasis is a common, chronic, and inflammatory skin disease with a high burden on individuals, health systems, and society worldwide. With the immunological pathologies and pathogenesis of psoriasis becoming gradually revealed, the therapeutic approaches for this disease have gained revolutionary progress. Nevertheless, the mechanisms of less common forms of psoriasis remain elusive. Furthermore, severe adverse effects and the recurrence of disease upon treatment cessation should be noted and addressed during the treatment, which, however, has been rarely explored with the integration of preliminary findings. Therefore, it is crucial to have a comprehensive understanding of the mechanisms behind psoriasis pathogenesis, which might offer new insights for research and lead to more substantive progress in therapeutic approaches and expand clinical options for psoriasis treatment. In this review, we looked to briefly introduce the epidemiology, clinical subtypes, pathophysiology, and comorbidities of psoriasis and systematically discuss the signaling pathways involving extracellular cytokines and intracellular transmission, as well as the cross-talk between them. In the discussion, we also paid more attention to the potential metabolic and epigenetic mechanisms of psoriasis and the molecular mechanistic cascades related to its comorbidities. This review also outlined current treatment for psoriasis, especially targeted therapies and novel therapeutic strategies, as well as the potential mechanism of disease recurrence.
Similar content being viewed by others
Introduction
Psoriasis is a common, chronic, and inflammatory skin disease affecting approximately 2–3% of the global population. It has been reported that the incidence of psoriasis varies from 30.3 per 100,000 person-years to 321.0 per 100,000 person-years and is associated with age, sex, geographical location, ethnicity, and other genetic and environmental factors.1,2,3 Psoriasis can occur at any age, especially in two age group of 16–22 and 55–60 years. It also can significantly impact the quality of life and sources of income for the patients.2,4 An epidemiological study suggested that severe psoriasis could increase the loss of work productivity by more than four times, which is associated with a greater impact on quality of life (DLQI > 10), younger age (<40 years), and joint involvement.4 The severe economic burden has been found in multiple countries around the world and is correlated with the severity of the disease.5,6,7,8 Patients with psoriasis are also found to be significantly more prone to suicidal ideation (OR = 2.05, 95% CI: 1.54–2.74), suicide attempt (OR = 1.32, 95% CI: 1.14–1.54), and completed suicide (OR = 1.20, 95% CI: 1.04–1.39).9,10 In brief, psoriasis is a prevalent and costly disease that imposes a significant burden on individuals, health systems, and society worldwide.
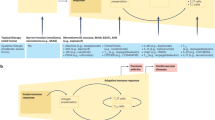
The etiology of psoriasis is complex and still remains largely unclear; it involves genetic susceptibility, environmental triggers, and immune dysregulation.11,12,13 Psoriasis is a heterogeneous disease that can be classified into different clinical types, with the most common type being plaque psoriasis (also known as psoriasis vulgaris), which causes dry, itchy, and raised skin patches (i.e., plaques) with silvery scales.14,15 These plaques can occur at any site of the skin but are more common on the elbows, knees, scalp, and lower back. Other types of psoriasis include guttate psoriasis, inverse psoriasis, pustular psoriasis, and erythrodermic psoriasis (Fig. 1).12,16,17,18,19 A growing body of research has indicated that psoriasis is a systemic disease with a variety of comorbidities, such as cardiovascular diseases, metabolic syndrome, psoriatic arthritis, depression, and anxiety.11,20,21,22 Therefore, attention needs to be given to comorbidities of psoriasis as well as the disease per se to work out early intervention and treatment strategies.
To date, the diagnosis of psoriasis is mainly based on the clinical features of skin lesions, such as morphology, location, and scaling status. A skin biopsy can be performed to confirm the diagnosis or exclude other conditions.23,24,25 The most common microscopic features of psoriasis vulgaris are hyperkeratosis with parakeratosis and neutrophil microabscesses in the parakeratosis region (also known as Munro’s microabscesses). It is also characterized by significantly reduced or absent granular layer, thinning of the muscular layer above the dermal papilla, extending or shifting telangiectasia, and infiltration of lymphocytes and neutrophils around the lesions.12,26,27 Psoriatic arthritis can be diagnosed by a rheumatologist through clinical examination, medical imaging, and laboratory tests.28,29 The treatment of psoriasis depends on the severity, extent, and type of psoriasis, as well as the patient’s preferences and comorbidities. The primary goals of treating psoriasis are to reduce inflammation, clear skin lesions, improve quality of life, and prevent complications. Conventional therapies for psoriasis include corticosteroids, vitamin D analogs, calcineurin inhibitors, phototherapy, methotrexate, cyclosporine, acitretin, and apremilast.11,24,28 During the past decade, injectable biologics targeting specific molecules involved in the pathogenesis of psoriasis have gradually been developed or studied. They include TNF-alpha inhibitors (adalimumab, etanercept, infliximab), IL-12/23 inhibitors (ustekinumab), IL-17 inhibitors (secukinumab, ixekizumab), and IL-23 inhibitors (brodalumab), and so on.30,31
In this review, we looked to summarize and discuss the signaling pathways involved in psoriasis and their roles, the clinical diversity of psoriasis and associated comorbidities, pathophysiology, epidemiology, targeted therapies, as well as clinical research progress. We hope to provide a reference for future studies on and personalized treatment of psoriasis.
Signaling pathways driving psoriasis pathogenesis
The pathogenesis of psoriasis has gradually been elucidated in the past years. Studies have revealed that psoriasis is regulated by the complex interactions between extracellular cytokine pathways and intracellular signaling molecules.32 A variety of cytokines transmit extracellular signals to the cell membrane and then are recognized by related receptors, leading to the activation of intracellular signaling pathways and inducing a series of events that ultimately result in the inflammatory signaling cascade.33,34 Epigenetics also plays a critical role in the pathogenesis of the psoriasis.35,36 Furthermore, recent studies also suggested that metabolic reprogramming is emerging as another crucial regulatory paradigm for psoriasis.37,38,39
Extracellular cytokine pathways
The TNF/IL-23/IL-17 pathway
The findings of a large number of genome-wide association studies (GWAS) and clinical trials support the central role of TNF-IL-23-IL-17 signaling pathways in the pathogenesis of psoriasis, especially for plaque psoriasis.40,41,42,43,44,45,46,47
IL-23
Interleukin-23 (IL-23) is a heterodimeric protein constituted by the p19 and the p40 subunits via a disapplied bond.48 Prior studies have revealed that IL-12 and IL-23 share the p40 subunit; however, only IL-23 uses the p19 subunit, while IL-12 uses the p35 subunit. Both IL-23 and IL-12 belong to the IL-12 family.49 IL-12 and IL-23 are mainly secreted by dendritic cells (DCs), the levels of which are elevated in psoriasis. A growing body of evidence has suggested that the RNA expression of both p40 and p19 increases greatly in psoriatic lesions, while the expression of p35 mRNA remains largely unchanged.50,51 The level of IL-23 protein can be greatly elevated in psoriatic lesions compared to unaffected skin.50 Thus, it can be inferred that the pathogenesis of psoriasis is related to IL-23 rather than IL-12.
IL-23 acts on T cells, especially the CD4+ helper T cells (Th17 cells), via a cellular receptor complex comprising of two transmembrane proteins: IL-23Rα and IL-12Rβ1.52 Then, IL-23 promotes the release of interleukin-17 (IL-17), another critical cytokine involved in the pathogenesis of psoriasis, by Th17 cells through the activation of intracellular signals.53
IL-17
The IL-17 family includes six structurally related cytokines: IL-17A to IL-17F.54 Among them, it is reported that IL-17A, IL-17C, and IL-17F are related to the pathogenesis of psoriasis due to their increased expression in psoriatic lesions.55,56,57 Although IL-17C and IL-17F exhibited higher levels of expression in psoriasis, IL-17A is found to be the most biologically active cytokine in this family.55,58 IL-17A and IL-17F can function as homodimers or IL-17A/F heterodimers.59,60 To date, IL-17A (commonly known as IL-17) has received the greatest attention due to its pro-inflammatory role in autoimmune disease.61 Although it is mainly produced by Th17 cells. Recently, innate immune cells, including ILC3 cells, γδT cells, neutrophils, and mast cells, have also been found to source IL-17 in psoriasis.62
Similar to IL-23, IL-17 also acts on its target cells through corresponding receptor complexes. The signals of IL-17A/IL-17A and IL-17F/IL-17F homodimers and IL-17A/IL-17F heterodimers transmit via IL-17RA and IL-17RC transmembrane complex. The IL-17RA chain is also the co-receptor of IL-17C (when binding to IL-17RE) and IL-17E (when binding to IL-17RB).63 A recent study has revealed that CMTM4 is a new component of the IL-17RC. It can mediate the plasma membrane localization of IL-17RC and the subsequent transduction of IL-17A and IL-17F signaling.64 The released IL-17, especially IL-17A and IL-17F, mainly acts directly on keratinocytes to stimulate the production of some molecules such as cytokines, antimicrobial peptides (AMPs), and β-defensins, as well as chemokines, including chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, CXCL8, and CCL20; these molecules are often increased in psoriatic lesions to attract neutrophils, macrophages and lymphocytes.65 There is also research finds IL-17A can upregulate Galectin-8, which further promotes keratinocytes proliferation by regulating its mitosis.66 S100A8 and S100A9, as reliable biomarkers of psoriasis disease activity, are highly up-regulated in keratinocytes. It has been investigated that IL-17A and IL-17F can highly induce S100A8 and S100A9 expression and release.67 Moreover, IL-17 can promote keratinocyte stemness and then promote keratinocyte proliferation.68 Except for acting on keratinocytes, recent studies have revealed that IL-17A can also be involved in psoriasis via regulating other stromal cells, T cells, or monocytes. On the one way, IL-17A directly induces IL-19 and IL-24 expression of fibroblast cells in the skin via binding with IL-17RA and IL-17RC, which further contributes to keratinocyte proliferation and acanthosis.69 On the other way, the released IL-17A also acts on other immune cells to facilitate psoriasis pathogenesis. Liu et al. have found that IL-17A can block the suppressive function of Tregs.70
Unlike IL-17A and IL-17F, IL-17C is primarily released by keratinocytes. Previous studies have suggested that IL-17C is produced by keratinocytes after stimulation by TNF-α or toll-like receptor agonists and then exerts the effects of IL-17A and IL-17F.71 IL-17C can also stimulate the production of human β-defensin 2 and granulocyte colony-stimulating factor (Fig. 2).72 As for IL-17E, it acts as a crucial factor in recruiting innate immune cells like monocytes/macrophages and neutrophils to the skin. What’s more, it targets keratinocytes in an autocrine manner and then induces keratinocyte proliferation via the mTOR signaling pathway in psoriasis.73
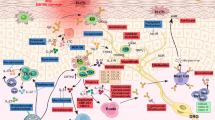
The cross-talk of extracellular cytokine pathway in psoriasis. A complex network links the essential cells and molecules in the pathogenesis of psoriasis. And the cross-talk is considered the core of the progress. On the one hand, the mutual promotion of adaptive and innate immune system produces several cytokines and maintain psoriatic hallmark features in both the dermis and epidermis. On the other hand, the keratinocytes facilitate the inflammatory mediators and enhance the expansion of local activation. Created with BioRender.com. Abbreviations: MCs mast cells, pDCs plasmacytoid dendritic cells, mature DCs mature dendritic cells, MCP Monocyte chemotactic protein, CCL20 Chemokine (C-C motif) ligand 20
TNF-α
Tumor necrosis factor (TNF)-α is another critical inflammatory cytokine that is highly expressed in psoriatic lesions. This cytokine plays a pivotal role in the pathogenesis of psoriasis, which is also demonstrated by the efficacy of TNF-α-targeted therapies.74,75 TNF-α is produced by a variety of cells related to the development of psoriasis, such as keratinocytes, DCs, neutrophils, mast cells, as well as NKT, Th1, Th17 and Th22 cells.76,77 It acts on the targeted cells mainly via two types of TNF receptors, i.e., TNFRI (p55) and TNF-RII (p75). It has been reported that TNF-α exerts its biological effect on epidermal cells by binding to the p55 TNF-R.78On the one hand, TNF-α significantly suppresses the secretion of IFN-α of plasmacytoid dendritic cells (pDCs).79 It is the main reason for the occurrence of aggravated psoriasis or new paradoxical psoriasis during treatment with TNF-α inhibitors.80 On the other hand, TNF-α favors pDCs maturation to a more conventional dendritic cell phenotype to produce IL-2381; Moreover, TNF-α can induce the synthesis of IL-12 and IL-18, two cytokines that are potent inducers of IFN-γ, to participate in the regulation of the Th1 immune response.82 Furthermore, TNF-α acts synergistically with IL-17A to coregulate psoriasis-related cytokines and keratinocyte genes, affecting the function of keratinocytes.83 All of the above findings suggest that TNF-α is an important regulator of the IL-23/IL-17 axis. Recently, research reveals that TWEAK displayed a similar effect with TNF-α and IL-17A on up-regulating the expression of CXC chemokines, along with cytokines such as IL-23 and inflammation-associated proteins like S100A8/9. Moreover, it may act with TNF and IL-17 to enhance the feedback inflammatory activity of keratinocytes. Hence, TWEAK inhibition may be comparable to targeting TNF or IL-17. Blocking TWEAK may be considered an alternate therapy for psoriasis.84
As can be seen, on the one way, the released TNF-α facilitates the release of IL-23 by DCs. On the other way, TNF-α also interacts with keratinocytes to release inflammatory cytokines and promote T-cell infiltration. Then, the secreted IL-23 acts on T cells, especially Th17 cells, to promote the release of IL-17 by binding to the IL-23 receptors. Furthermore, IL-17 acts synergistically with TNF-α to stimulate keratinocytes to produce inflammatory cytokines and chemokines to recruit more inflammatory cells, thus amplifying the inflammatory cascade, leading to the development of psoriasis. The central role of the TNF-α/IL-23/IL-17 axis in the pathogenesis of psoriasis has been demonstrated in numerous studies (Fig. 2).
The CCL20-CCR6 pathway
CCL20, also known as MIP-3α or LARC, is an important chemokine in psoriasis. Unlike other chemokines that have multiple receptors, CCL20 binds to only one receptor, CCR6.85 It is observed that CCL20 is increased in the serum and lesions of patients with psoriasis,86 which has been further confirmed in an in vivo study showing the upregulation of CCL20/CCR6 in psoriasis mouse models. It is found that the level of CCL20 and CCR6+T cells increases greatly in the imiquimod-induced psoriasis mouse model and that CCR6+ γδ T cells infiltrate in the skin producing IL-17A and IL-22 in the IL-23-induced psoriasis model.87,88 Thus, it seems that the CCL20/CCR6 axis plays a vital role in the pathogenesis of psoriasis.
The major source of CCL20 is keratinocytes, while CCR6 is mainly expressed in DCs and T cells, especially Th17 cells.89 A recent study suggested that CCR6 is a representative maker for Th17 cells.90 It is found that keratinocyte transglutaminase 2 (TG2) can upregulate CCL20 expression, promoting the recruitment of CCR6+ γδ T cells.91 Moreover, CCL20 can be upregulated by several cytokines, such as IL-17A and TNF-α, in keratinocytes.65,92 Cellular dissociation by scratching or trypsinization is also found to be a potent stimulator for the rapid production of CCL20 in keratinocytes.93,94
In brief, external factors such as scratching or cytokines can stimulate the production of CCL20 by keratinocytes in psoriatic lesions. Subsequently, the released CCL20 recruits CCR6+ Th17 cells, which produce IL-17A, further enhancing the selection of CCL20. Thus, the CCL20/CCR6 axis works as a driving force in the maintenance of the TNF-α/IL-23/IL-17A axis (Fig. 2).
The IL-36/IL-1 pathway
In addition to the TNF-α-IL-23-IL-17 axis, the IL-36-IL-1 inflammatory axis is also predominant in the pathogenesis of psoriasis, especially the generalized pustular psoriasis (GPP).95
IL-36 comprises of three agonists, namely IL-36α, IL-36β, and IL-36γ, and two antagonists, i.e., the IL-36 receptor antagonist (IL-36RN) and IL-38.96 IL-36 belongs to a subgroup in the wider IL-1 family,97 which also include IL-1α, IL-1β, IL-1 receptor antagonist (IL-1RN), IL-18, IL-33, and IL-37.98 Johnston et al. have reported that the expression of IL-36α, IL36β, IL-36γ, and IL-36Ra is up-regulated in psoriasis and can further promote the expression of keratinocyte antimicrobial peptide, indicating their importance in psoriasis.99 Moreover, the identification of mutations in IL-36 signature genes, especially the IL-36RN gene, confirmed the pathogenic role of the IL-36 axis in the development of psoriasis.100,101,102 In 2015, IL-1RL1 was identified as a new susceptibility locus for psoriasis.103 Further genetic studies indicated that polymorphisms of the IL-1B gene could be used to distinguish between early- and late-onset psoriasis.104
Keratinocytes have been identified as the predominant source of IL-36, which acts on target cells through the receptor, IL-36R (also known as IL-1RL2 or IL-1Rrp2).105 It has been demonstrated that the IL-36 cytokines need the N-terminal peptide cleavage to activate.106 The activation can be carried out by the serine proteases, including cathepsin G, elastase, and proteinase 3, which are derived from neutrophils. Besides, it can also be activated by cathepsin S released from keratinocytes and fibroblasts.107,108 During this progress, the activation of IL-36 can be inhibited by protease inhibitors, including α1-antitrypsin and α1-antichymotrypsin. More interestingly, α1-antitrypsin and α1-antichymotrypsin are encoded by SERPINA1 and SERPINA3, and the latter is one of the most common mutations molecular in pustular psoriasis.95 Further studies have shown that activating IL-36 promotes the production of neutrophil-attracting chemokines (CXCL1, CXCL2, CXCL8), other T cell chemokines, macrophage chemokines, and antimicrobial peptides; this process seems to be a self-amplifying loop.109,110 Moreover, mature IL-36 induces T-cell proliferation and Th-1 and Th-17 cell differentiation.111,112 In addition, the activated IL-36 also acts on dendritic cells to support their phenotypic and functional maturation and trigger the production of IL-1β, TNF-α, and IL-23.32,113 Recent research has investigated that a novel soluble IL-36R (sIL-36R), which is encoded similar ectodomain of IL-36R, can suppress psoriasis inflammation by binding IL-36γ and competing with IL-36R for IL-36γ binding in a dose-dependent manner. The production of sIL-36R is mainly regulated by the IL-36 pre-mRNA splicing mediated by DDX5. Moreover, the activated IL-17D signaling inhibits the expression of DDX5. This study provides new insight into how a novel soluble IL-36R participates in psoriasis inflammation.114
IL-1β is the main form of IL-1 in circulation. Like other cytokines in the IL-1 family, it has an inactive precursor that requires the caspase-1 cleavage or neutrophil-derived serine proteases to be biologically activated.115 DCs, monocytes, and macrophages are the main sources of the expression, activation, and secretion of IL-1β. It is reported that the level of IL-1β expression is elevated in psoriatic lesions and correlated with the severity of disease and treatment response. IL-1β has been demonstrated to play a critical role in the differentiation of Th17 and γδT17 cells. It can also stimulate keratinocytes to secrete chemokines such as CCL20, which is an important chemokine increase in psoriasis (Fig. 2).116
The IFN pathway
There are three subtypes of IFNs, i.e., type I (IFN-α and IFN-β), type II (IFN-γ), and type III (IFN-λ).117 IFNs of type I and type III can be released by several types of cells, such as epithelial cells, macrophages, and DCs, while the type II IFN is secreted by activated NK and T cells only.118,119 Recently, it has been highly recognized that IFNs serve as vital mediators in the pathogenesis of psoriasis, with type I and type II IFNs being the main mediators. Type I IFNs are key cytokines related to chronic viral infection, which often triggers psoriasis.120 Thus, treatment with type I IFNs often induces or exacerbates psoriasis or psoriatic arthritis.121,122 Preclinical experiments also found that mice lacking type 1 IFN receptor or treated with IFN-α or IFN-β antibodies failed the treatment for Th17-mediated psoriasis-like inflammation.123 Previously, psoriasis was regarded as an IFN-γ-mediated disease due to the significant IFN-γ genomic signature in psoriatic lesions.124 The main IFN-γ-producing cells, Th1 cells, are also considered an integral part of the pathogenesis of psoriasis.125 Prior studies have found a positive correlation between serum IFN-γ levels and the severity of disease assessed using the Psoriasis Area and Severity Index (PASI). Hence, IFN-γ seems to be a prognostic factor of this disease.126 All of the above findings indicate that the IFN pathway plays a critical role in the pathogenesis of psoriasis.
Studies have shown that the innate immune cells such as skin-resident keratinocytes and infiltrated plasmacytoid dendritic cells (pDCs) play a significant role in initiating subsequent adaptive immune events, including DCs maturation and T cell activation.127,128 Gilliet et al. found that pDCs are the natural IFN-α producing cells; pDCs infiltrate the skin and are activated to produce IFN-α at the early stage of psoriasis.129 With regard to IFN-β, numerous studies have recognized keratinocytes as the primary source of this cytokine.130 Along with IFN-α, IFN-β can also trigger the maturation and differentiation of conventional dendritic cells (cDCs) via IFNAR1 and IFNAR2 receptors on the target cells.131 Subsequently, the mature DCs release cytokines such as TNF-α, IL-23, and IL-1β to activate Th17 cells. Moreover, IFN-α can increase the expression of IL-22 receptors on keratinocytes, enhancing the response of keratinocytes to IL-22.132
The main source of IFN-γ is activated Th-1 cells. Besides, IFN-γ can also be secreted by DCs, CD8 T cells, and NK cells.133 The secreted IFN-γ activates their effect cells by binding IFN-γ receptors (IFNGRs), which are composed of two transmembrane chains: IFN-γ R1 and IFN-γ R2.133 The production of IFN-γ can be regulated by IL-12, IL-18, and IL-23. Moreover, IL-23 has a synergistic effect with IL-18 for IFN-γ production.134,135 In psoriasis, IFN-γ acts on keratinocytes, leading to the activation and production of antimicrobial peptides such as LL-37 cathelicidin and β-defensins.125 Recent studies also found that IFN-γ could induce the release of cytokines (IL-23 and IL-1) and adhesion molecules of DCs, which activate the Th17 cells.136
In summary, the IFN pathway plays an important role in the inflammatory cascade in the pathogenesis of psoriasis (Fig. 2).
Other cytokines and pathways related to psoriasis
IL-22
IL-22 belongs to the IL-10 family, which also includes IL-10, IL-19, IL-20, IL-24 and IL-26.137 Prior studies have found that the level of IL-22 is significantly increased in the serum and lesions of patients with psoriasis.138,139 The concentration of serum IL-22 is also significantly correlated with the PASI score and is regarded as an indicator of therapeutic effect, as treatment with etanercept or methotrexate can reduce the level of serum IL-22. This also indicates that serum IL-22 is a potential predictor for the severity of psoriasis.140,141
IL-22 can be secreted by a variety of immune cells, such as Th17 cell, Th1 cell, Th22 cell, γδT cell, and NK cells.142,143,144 The secreted IL-22 acts on its target cells by binding to IL-22 receptors, which consist of two subunits, IL-22R1 and IL-10R2 (also known as IL10RB).145 It has been demonstrated that IL-22 does not act on immune cells because IL-22R1 is only expressed on non-immune cells such as keratinocytes.146 The binding of IL-22 to IL-22R leads to the activation of downstream signals (which will be presented in the next section) in keratinocytes, inducing the production of antimicrobial proteins.146 Furthermore, IL-22 can inhibit the differentiation of keratinocytes, leading to acanthosis, a typical psoriasis-like inflammation of epidermis.147 Interestingly, TNF-α can enhance this effect of IL-22.147 However, the therapeutic inhibition of IL-22 might be insufficient for a good clinical response according to early clinical trials on anti-IL-22 monoclonal antibodies (ILV-095 and ILV-095) in patients with psoriasis, which were discontinued for their failure to meet the primary efficacy endpoint (ClinicalTrials.gov identifier: NCT00563524 and NCT01010542).
IL-6
IL-6, a traditional cytokine in psoriasis, may exert a controversial effect on psoriasis. During the past two decades, IL-6 has been reported to be associated with the pathogenesis of psoriasis.133,148 But only till recently, the expression of the IL-6 gene has been regarded as a marker for the pathological state of psoriasis and psoriatic arthritis.149 In psoriasis, the source of IL-6 includes almost all stromal cells, including keratinocytes, fibroblasts, endothelial cells, and immune cells such as dendritic cells, macrophages, and Th17 cells.150,151 IL-6 acts through interacting with IL-6R. The fully competent IL-6R consists of an alpha subunit IL-6R (CD126) and a commonly expressed beta subunit gp130 (CD130).152,153 Recent evidence demonstrates that IL-6 can initiate the development of Th17 cells.154 The IL-17 generated by Th17 cells can feedback regulate the production of IL-6 of keratinocytes.83 The positive feedback loop of IL-6/IL-17 signaling participates in the proinflammatory interaction of innate and adaptive immunity in patients with psoriasis. Moreover, IL-6 can promote neutrophil migration by increasing the level of chemokines released by mononuclear cells/macrophages, such as IL-8 and MCP-1.153 Mature neutrophils also release pro-inflammatory cytokines such as IL-23 and IL-17 by responding to IL-6 through the membrane-bound IL-6R, which helps to establish a positive feedback loop for Th17 polarization.148 Based on these findings, IL-6 might serve as a pathogenic factor for psoriasis, and the blockade of IL-6 might bring benefits to patients with this disease. Most evidence to date, however, suggests otherwise. Tocilizumab, a humanized anti-IL-6 receptor monoclonal antibody, has been approved by the FDA since 2010 for the treatment of moderate-to-severe rheumatoid arthritis (RA). However, in some cases, tocilizumab appeared ineffective for psoriatic arthritis and even induced or exacerbated psoriasis;155 this might be explained by the absence of IL-6 leading to excessive production of additional proinflammatory cytokines by keratinocytes.156 These findings may provide a new insight into why the blockage of IL-6 was ineffective in the treatment of psoriasis and why some patients with RA treated with IL-6 inhibitors had newly onset psoriasis.
IL-9
There are numerous other cytokines and pathways acting as a promoter in the pathogenesis of psoriasis, such as IL-9. The level of IL-9 has been found to be increased in both the serum and the lesions of patients with psoriasis and positively correlated with the severity of psoriasis and impaired quality of life (QoL).157 Cells producing IL-9 (the Th9 cells) are also found increased in the skin lesions of psoriasis.158 IL-9 can promote the secretion of IL-17, IL-13, IFN-γ, and TNF-α in psoriasis.158 However, the mechanisms of how IL-9 regulates the microenvironment of psoriasis still need further research.
IL-33
IL-33, also known as “alarmin” and belonging to the IL-1 family, is also reported to be increased in psoriatic lesions.159 IL-33 is mainly produced by non-hematopoietic cells such as keratinocytes and fibroblast cells, but is also expressed by hematopoietic cells, especially mast cells (MCs) and DCs.160 In psoriasis, MCs can be activated by IL-1 and then produce TNF and IL-33, the latter in turn activating MCs in psoriatic inflammation.161 The secreted IL-33 also binds to its receptor, ST2 receptor, to promote the proliferation of keratinocytes, which aggravates the psoriasis. Interestingly, more and more findings have suggested that IL-33 exerts an immunosuppression effect by inhibiting the differentiation and function of Th17 cells.162 Hence, more studies are needed to explore the comprehensive role of IL-33 in psoriasis.
Intracellular signaling pathways of transmission
The NF-κB pathway
One of the features of psoriasis is the elevated level of active, phosphorylated nuclear factor kappa B (NF-κB).128 NF-κB, a protein transcription factor, is a key regulatory element involved in a variety of immune and inflammatory pathways. The NF-κB pathway plays a crucial role in psoriasis, as it was found to alter the behaviors of keratinocytes and immune cells by affecting their proliferation, differentiation, and production of cytokines or chemokines. NF-κB and its related pathways are also found to be predictive of the patient’s clinical outcome.163,164 NF-κB is activated through two pathways: the canonical and non-canonical pathways. The canonical NF-κB pathway usually responds to a variety of stimuli, including ligands of various cytokine receptors, members of the TNF receptor (TNFR) superfamily, pattern-recognition receptors (PRRs), T-cell receptors (TCR), and B-cell receptors.165 The major mechanism for canonical NF-κB activation is the polyubiquitination of the multi-subunit IκB kinase (IKK) complex, which induces degradation of IκBα, resulting in transient nuclear translocation and activation of p50/RelA and p50/c-Rel dimers. The IKK complex consists of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NF-κB essential modulator (NEMO, also known as IKKγ).166,167 The polyubiquitination of IKK is mainly mediated by tumor necrosis factor-associated factors (TRAFs) or E3-ligases. TRAFs, especially TRAF6, catalyze the ubiquitination of adaptor proteins such as ACT1, leading to the phosphorylation of protein kinases, including IKKs.168 It is the canonical NF-κB pathway that plays an important role in psoriasis pathogenesis. With regard to the non-canonical pathway, its activation relies on the phosphorylation of p100, which is induced by NF-κB-inducing kinase (NIK). The non-canonical pathway selectively responds to specific members of the TNF cytokine family, such as CD40 ligand and B-cell activating factor (BAFF).167,168
Genomic studies have revealed that numerous genes encoding components in the NF-κB pathway are linked to the development of psoriasis. GWAS have found that c-Rel, a gene encoding one of the members in the NF-κB family, is related to psoriasis susceptibility.169,170 Downregulation of c-Rel can inhibit the growth of keratinocytes by affecting their cell cycle progression.171 IL-23 is regarded as a direct target of c-Rel, and a decreasing level of c-Rel can also inhibit the production of IL-23.172 Thus, the susceptibility gene of c-Rel might be a promising target to treat and prevent psoriasis. TRAF3 interacting protein 2 (TRAF3IP2), which encodes Act1, is identified as a novel susceptibility gene for psoriasis and psoriatic arthritis by another GWAS.41 Act1 mediates the activation of NF-κB and its related pathway by interacting with TRAF6, a member of the TRAF family, resulting in increased production of inflammatory factors.173,174 Of note, CARD14 (also known as CARMA2), a proinflammatory signaling molecule, is usually expressed in several types of skin cells, such as keratinocytes, dermal γδ T cells, and Langerhans cells.175,176 Studies have found that CARD14 is located in the psoriasis susceptibility locus 2 (PSORS2) and detected over 20 variations of CARD14 in psoriasis, indicating its vital role in the pathology of psoriasis.177,178,179 Mounting evidence has suggested that the spontaneous psoriasis-like inflammation is caused by the gain-of-function mutation of CARD14.180,181 CARD14 interacts with TRAF6 and ACT1, leading to constitutive activation of the NF-κB pathway and the subsequent production of several cytokines and chemokines related to psoriasis.180 Apart from the activators of NF-κB, several genes involved in the inhibition of NF-kB pathway were also found to be associated with psoriasis. Among them, NFKBIA, which encodes the NF-κB inhibitor IκB, was identified as a psoriasis susceptibility locus in 2010.169 Tumor Necrosis Factor Alpha-Induced Protein 3 (TNFAIP3, also known as A20) and TNFAIP3 Interacting Protein 1 (TNIP1) are also two of the first discovered genes associated with psoriasis.182 TNFAIP3 usually works together with TNIP1 to inhibit the NF-kB pathway by inhibiting the polyubiquitination of NEMO (also known as IKKγ), thereby preventing the degradation of the inhibitor molecule IκB.183,184 In addition, TNIP1 blocks the conversion of p105 to NF-κB subunit p50, leading to the decrease of NF-κB.184 ZC3H12C (encoding MCPIP3) also plays an important role in suppressing NF-κB signaling. A published GWAS has identified an SNP in ZC3H12C that is highly correlated with psoriasis.178 It is revealed that ZC3H12C can decrease TNFα-induced IKKα/β, IκBα phosphorylation, and p65 nuclear translocation.185,186
The JAK-STAT and TYK2 pathways
The Janus kinase-signal transducer and activator (JAK-STAT) of the transcription pathway plays a vital role in the intracellular cytokine signaling of a variety of cellular processes, and both of them are mediated by the immune system in normal and pathological states. The JAK family consists of 4 members, namely JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2).187 JAKs bind to type I and II cytokine receptors to transmit extracellular cytokine signals into cells to activate the STATs, leading to proinflammatory cellular immune responses.188 JAKs mediate the signaling of IL-12, IL23, IFN-α, IFN-β and IFN-γ, IL-6 and IL-22 and other cytokines.188 Among the JAKs, TYK2 mediates the signaling downstream of IL-23, IL-12, and type I IFN receptors.189 The cytokines bind to their receptors, activating receptor-associated JAKs. The activated JAKs can phosphorylate the STAT proteins, leading to STAT dimerize (mostly heterodimers) and translocation to the nucleus to regulate gene transcription.190
As discussed above, numerous major pathogenic mediators of psoriasis are linked to the JAK-STAT signaling pathway. JAK1 is directly related to the severity of psoriasis,191 and the deletion of TYK2 can suppress psoriasis phenotypes in different mouse models, suggesting their potential therapeutic value for psoriasis.192 JAK1, JAK2, and TYK2 are primarily involved in psoriasis due to their presence in all tissues. All three forms of JAKs activate STAT3, resulting in the activation and differentiation of Th17 cells. The JAK1/JAK2-dependent process also participates in the phosphorylation of STAT1, which leads to the activation of IFN-α/β.193 The increased expression of STAT1 and STAT3 was also found in psoriatic skin lesions compared to normal skin.194,195 More interestingly, genetic linkage studies have revealed an association between TYK2 mutations and psoriasis susceptibility.196
The MAPK pathway
Mitogen-activated protein kinases (MAPKs) are a family of highly conserved serine-threonine protein kinases that engage in signal transmission through a three-level cascade. The MAPKs family includes p38 MAPK, extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK).197 Each MAPK signaling pathway comprises at least three components, including a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK), and a MAPK. MAPKKKs phosphorylate and activate MAPKKs, which further phosphorylate and activate MAPKs.198 It has been demonstrated that ERK1/2, p38, and JNK MAPK are activated and increased in psoriatic lesions, indicating that the MAPK pathway is involved in the pathogenesis of psoriasis.199,200,201
Each MAPK pathway plays an important role in psoriasis. Many signals (e.g., DAMPs, CCN1, and IL-22) in psoriasis can activate the JNK pathway in keratinocytes. The activated JNK pathway in keratinocytes can mediate the recruitment of immune cells in psoriasis by regulating the production of inflammatory cytokines/chemokines, such as IL-6, IL-8, IL-23, IFNγ and TNFα, and CCL20 and hβD-2. In addition to the influence on the proliferation and differentiation of keratinocytes, this activated pathway is also involved in the recruitment and activation of Th1 or Th17 cells to produce additional cytokines such as IL-17, IL-22, and hβD-2.202,203 JNK is also recognized as a key factor regulating FOXP3, thereby moderating the development and maturation of Tregs.204 It should be noted that, apart from the NF-κB signaling, CARD14 also activates the JNK and p38 MAPK pathways and induces the production of inflammatory cytokines.205
The P38 MAPK pathway also plays a crucial role in psoriasis. It has been found that the production of S100A8 induced by the IL-17 and TNF-α is mainly regulated by a P38-dependent mechanism.206 Similarly, the production of hBD-2, hBD-3, and S100A7 induced by TNF-α also relies on the phosphorylation of p38 MAPK in keratinocytes.207 CCN1 can promote the production of IL-1β in keratinocytes by activating the p38 MAPK signaling.208 Mitogen- and stress-activated protein kinase 1 (MSK1) is a downstream target of both p38 and ERK1/2 MAPKs. MSK1 regulates the expression of proinflammatory cytokine genes by activating transcription factors. A prior study found an increased level of phosphorylated MSK1 (Ser376) in psoriatic lesions.209 In psoriasis, a high level of IL-6 can lead to increased activation of p-ERK1/2.210 Keratin16 (KRT16), a hyperproliferation-associated keratin in keratinocytes, has proven to enhance keratinocyte proliferation and VEGF secretion by activating and phosphorylating the ERK signaling pathway.211 Furthermore, DUSP1/MKP-1, a negative regulator of the MAPK pathway, is found to be downregulated in psoriasis. The overexpression of DUSP1 can markedly inhibit keratinocyte proliferation and promote apoptosis by targeting the ERK signaling pathway.212
The PI3K-AKT pathway
The PI3K/Akt pathway has been regarded as an important mechanism in the occurrence and progression of psoriasis.213,214 A growing body of evidence has revealed that the activation of this pathway by external or internal stimulation can lead to epidermal hyperplasia, immune pathogenesis, angiogenesis, and other physiological or pathological processes related to psoriasis.215,216 Once activated by GFR tyrosine kinase, PI3K converts PIP2 into PIP3 on the plasma membrane. PIP3 binds to the pleckstrin homology (PH) domain of Akt, which enables phosphoinositide-dependent kinase 1 (PDK1) to phosphorylate Thr308 of Akt and PDK2 to phosphorylate Ser473. Once completely activated, Akt acts as the core molecule and moves to the cytoplasm and nucleus to regulate the pathogenesis and progression of psoriasis via the downstream signaling pathway.217 As downstream factors of Akt, both forkhead box O (FOXO) and mammalian target of rapamycin (mTOR) regulate the growth, survival, and proliferation of keratinocytes.218
FOXO transcription factors are negatively regulated by phosphorylated Akt, and activated FOXO can inhibit cellular proliferation.219 It has been demonstrated that the transcriptional activity of FOXO can be regulated by nuclear translocation.220 Mounting evidence has revealed elevated expression and activation of PI3K and AKT in the keratinocytes of psoriatic lesions.221,222,223 Higher expression of PI3K may cause Akt hyperactivity, further promoting the proliferation of keratinocytes through the phosphorylation of FOXO. Prior studies also found that FOXO is mainly expressed in the cytoplasm of psoriatic keratinocytes, while is present in the nucleus of skin cells unaffected by psoriasis and normal skin cells.224 Hence, excessive activation of P-Akt may change the location of FOXO from the nucleus to the cytoplasm, resulting in reduced inhibition of cell proliferation and hyperproliferation of keratinocytes.
MTOR includes two complexes, mTOR complex 1 (mTORC1) and mTORC2, both of which are essential for the progression of psoriasis due to their function of regulating cell proliferation and inflammation. Activated mTORC1 signaling can be detected in all epidermal layers in psoriasis. A series of cytokines, such as IL-1β, TNF-α, and IL-17A, or a combination of them, have proven to activate the mTORC1 signaling axis.223 IL-22 can also activate the Akt/mTOR pathway, promoting the growth of keratinocytes.225 Furthermore, studies have shown that the PI3K/Akt/mTOR pathway can regulate both innate and adaptive immune responses. This pathway is believed to be able to moderate the Th1/Th2/Th17 imbalance in psoriasis.215 After being activated by cytokines, mTOR is also involved in the secretion of proinflammatory molecules, such as CXCL8, IL-6, and VEGF, by keratinocytes.226 It has been suggested that mTORC2 might be essential for FOXP3 stability mediated by chemokine CCL3.227 Notably, overactivated mTORC1 contributes to parakeratosis (nuclei retention), which is a form of pathological change of psoriasis.228 In brief, the mTOR axis serves as a crucial regulator of inflammation and cell proliferation in psoriasis.
PTEN (phosphatase and tensin homolog deleted on chromosome 10) is a well-known tumor suppressor gene mutated in many human tumors.229 It is also a crucial upstream inhibitor of the PI3K/Akt pathway and has been recognized as a FOXO target gene since FOXOs can enhance PTEN transcription.217 Accumulating evidence has revealed that the expression of PTEN is reduced in psoriatic lesions, which might be a reason for the hyperproliferation of psoriatic keratinocytes.230 In psoriasis, the expression of PTEN can be regulated to adjust its effect on keratinocyte proliferation by a variety of factors, especially miR-RNA such as miR-233, miR-155, and miR-1228-3p.231,232,233
Other intracellular signaling pathway
In as early as 2010, it was indicated that altered Wnt signaling played an important role in psoriasis, as Wnt-5a was upregulated in psoriatic lesions, whereas WIF-1 was downregulated. A variety of cytokines, such as TGF-α, TNF-α, IFN-γ, and IL-1α, can upregulate the expression of Wnt-5a.234 Subsequent studies have found that Wnt-5a is a key regulator of keratinocyte proliferation in psoriasis.235 A recent study on the differential DNA methylation of miRNA-encoding genes in psoriasis highlighted the Wnt pathway. The comprehensive involvement of the Wnt pathway indicates that Wnt-mediated signaling not only contributes to the inflammatory condition of psoriasis but also underlies the disease susceptibility.236 However, further work on how the Wnt pathway participates in the inflammation and disease susceptibility of psoriasis is warranted.
Moreover, recent studies have identified a novel proinflammatory signaling pathway driven by cyclin-dependent kinase 4 (CDK4)/CDK6 and the methyltransferase EZH2.237 CDK4/6 phosphorylates EZH2 in keratinocytes, leading to a methylation-induced activation of STAT3. The activated STAT3 further increases the production of IκBζ, which is a key proinflammatory transcription factor required for cytokine synthesis and is induced in epithelial cells such as keratinocytes by IL-17 and polymorphisms in NFKBIZ (which encodes IκBζ and is recognized as one of the major susceptibility loci for psoriasis).238 Thus, treatment targeting the CDK4/6-EZH2 pathway is a promising strategy to tackle psoriasis.
Crosstalk between different pathways
In psoriasis, cytokines act on their target cells by binding to related membrane receptors to activate complex intracellular pathways. Among all the pathways, the NF-κB pathway, the MAPK pathway, and the JAK-STAT pathway are the most prominent ones to mediate cytokine signal transmissions across the cell membrane, while other pathways are often involved in some other STIM transmissions in psoriasis, including non-coding RNA (Fig. 3).
The comprehensive view of intracellular transmission pathway in psoriasis. There are mainly two kinds of transduction way in the psoriatic cytokine. a, b The one relies on JAK-STAT and TYK2 pathways to regulate gene transcription. Specifically, IFN-α and IFN-β activate STAT1/STAT2 via JAK1 and TYK2, while IFN-γ signaling activates STAT1/STAT1 dimerize via JAK1 and JAK2.The signal of IL-12 activates STAT4/STAT4 dimerize, while IL-6, IL-23, IL-22 activates STAT3/STAT3 dimerize. c The other relies on the different cytoplasmic complexes assemble to activate NF-κB/MAPK pathway. The transduction of TNF-α, IL-17, IL-1, and IL-36 is mainly via this. Created with BioRender.com. Abbreviations: IRF9 interferon regulatory factor 9, ISRE interferon-sensitive response element, GAS IFN-γ-activated site, ISGs IFN-stimulated genes, Blimp1 B lymphocyte induced maturation protein 1, RORγt retinoic acid receptor-related orphan receptor gamma t, MyD88 myeloid differentiation factor 88, IRAK interleukin-1 receptor-associated kinases, AP1 activating protein-1, TRAF TNF receptor associated factor, Ub ubiquitin, LUBAC linear ubiquitin chain assembly complex, TRADD TNFR1-associated death domain protein, RIPK1 receptor-interacting serine/threonine-protein kinase 1, cIAP1 cellular inhibitor of apoptosis protein 1, TAK1 TGFβ-activated kinase 1
As the central molecule in the immune microenvironment of psoriasis, IL-17A/IL17A, IL-17F/IL-17F homodimers, and IL-17A/IL-17F heterodimers mediate the ligation in initial subcellular events of IL-17RA/RC and downstream signals by recruiting and activating ACT1, TRAF6, and CARD14 complexes.180 TRAF6 further activates the NF-κB pathway by activating IKK, consequently leading to proteasomal degradation of phosphorylated IκB. In psoriasis, nuclear translocation of NF-κB and transcription of NF-κB target genes, such as AP-1 and C/EBPs, are increased.239 In addition to activating the NF-κB pathway, the signals can also activate the MAPK pathway (including JNK, ERK, and p38), and the released MAPKs phosphorylate and activate AP1.240 Of note, IL-17RA/RC can also recruit other signaling molecules, such as TRAF2 and TRAF5. Unlike TRAF6, TRAF2 and TRAF5 activate RNA-binding proteins rather than enhancing gene transcription, thus promoting mRNA stabilization.61
The JAK-STAT and TYK2 pathways play an important role in the signal transmission of cytokines in psoriasis, with different combinations of JAKs mediating different cytokine signaling. JAK2 couples with TYK2 to mediate the signal transduction downstream of IL-12 and IL-23 receptors. IL-12 binds to the IL-12 receptor to activate JAK1 and TYK2, further leading to STAT4 activation, thereby inducing the Th1 cell response. However, IL-23 usually activates STAT3 in Th17 cells, leading to subsequent induction of cytokines such as IL-17A and IL-17F.33,189 STAT3 also plays an important role in Th17 cell differentiation and keratinocyte proliferation via JAK1/JAK2 or JAK1/TYK2 signaling induced by IL-6.241 The phosphorylation and dimerization of STAT3 by IL-6 and IL-23 signaling enhance the expression and nuclear translocation of RORγt, further activating Th17 gene promoters, including Il17a, Il17f, Il23r, Csf-2, and Ccr6. Moreover, IL-23 signaling-induced transcription factor Blimp-1 enhances the function of Th17 by co-localizing RORγt and STAT-3 at the sites of Il17a, Il23r, and Csf-2 enhancers.242 In addition, IL-22 is also responsible for the hyperplasia and differentiation of keratinocytes and the production of anti-microbial peptides by activating STAT3 mediated by JAK1 and TYK2.146,243 STAT1 is responsible for the signal transduction of both type I and type II IFNs. IFN-α and IFN-β bind to their receptors, activating STAT1 via JAK1 and TYK2, while IFN-γ signaling exerts effects via JAK1 and JAK2.244 Thus, increased STAT1 can result in the production of numerous proinflammatory mediators and the activation and maturation of dendritic cells, which subsequently stimulates Th1 and Th17 cells.245
Homotrimers of TNF-α bind to two forms of TNFRs to induce intracellular signaling mainly by recruiting and assembling different cytoplasmic complexes, leading to the activation of different pathways and patient outcomes (including inflammation, cell apoptosis and survival, and tissue regeneration and host defense).246 In psoriasis, TNF-α mainly binds to TNFRI to activate downstream signals through complex I, which consists of TNFR1-associated death domain protein (TRADD), TRAF2, receptor-interacting serine/threonine-protein kinase 1 (RIPK1), cellular inhibitor of apoptosis protein 1 (cIAP1) or cIAP2, and linear ubiquitin chain assembly complex (LUBAC).247 Once activated, complex I recruits the transforming growth factor-beta (TGFβ) -activated kinase 1 (TAK1) to activate MAPKs, resulting in the activation of the transcription factor AP1; this complex also activates IKK via Lys63-linked ubiquitin, leading to the activation of the downstream NF-κB pathway and the activation of the transcription factor NF-κB.246,247 Both transcription factors can regulate proinflammatory gene transcription and immune cell proliferation.
As mentioned above, IL-36 acts on its target cells mainly through IL-1RAcP or IL-1Rrp2, while IL-1β acts on its targets mainly via IL-1R1. It has been found that IL-36γ significantly enhances the interaction between IL-1Rrp2 and the accessory protein IL-1RAcP, subsequently activating the intracellular signaling (including IKBζ/NF-κB, MAPKs, c-Jun, and STAT3 signaling) via the MyD88/IRAK1/IRAK2/TRAF6 mechanism;112,248 this is also the primary mechanism by which IL-1β activates MAPKs and NF-κB.112
Metabolites in the pathogenesis of psoriasis
Keratinocyte hyperproliferation is one of the hallmarks of psoriasis. It requires extensive energy-supplying substances such as glucose and maybe the main driver of metabolic changes in psoriasis.128,249 As psoriasis has been regarded as a systemic disease related to metabolic abnormalities, researchers strived to investigate the mechanism of how metabolic factors affect the physiology and pathophysiology of psoriasis holistically and systematically. With the advances in metabolomic and bioinformatic analyses, metabolism has been identified as an important factor for the pathogenesis of psoriasis. Multiple forms of cellular metabolism, such as glycolysis, tricarboxylic acid (TCA) cycle, lipid metabolism, and amino acid metabolism, participate in the regulation of keratinocyte as well as related immune cells (Fig. 4). Treatment targeting metabolic factors may also be a potential strategy for coping with psoriasis.250
Metabolites in the pathogenesis of psoriasis. Compilation of the main disrupted metabolites and metabolic enzymes (red color) in psoriasis and their interconnections. Created with BioRender.com. Abbreviations: PA phosphatidic acid, DAG diacylglycerol, PE phosphatidylethanolamine, PC phosphatidylcholine, PS Phosphatidylserine, VLDL very low-density lipoproteins, HDL high-density lipoprotein, IDL intermediate-density lipoproteins, LDL low-density lipoproteins, HK2 hexokinase 2, PKM2 pyruvate kinase M2, PUFA polyunsaturated fatty acids, BCAAs branched-chain amino acids, SDH succinate dehydrogenase, GLS glutaminase, EAAs essential amino acid, ETC electron transport chain, AMPK AMP-activated protein kinase, ATP adenosine triphosphate, ADP adenosine diphosphate, AMP adenosine monophosphate, ROS reactive oxygen species
Metabolites of glycolysis
Glucose is the primary bioenergy source for rapidly proliferating cells, accelerating glycolysis and the TCA cycle. Recent studies found that pro-inflammatory cytokines can stimulate glucose uptake and glycolysis in human keratinocytes.251 Glucose uptake involves glucose transporters.252 Studies have suggested that the expression of glucose transporter-1 (GLUT-1) is upregulated in psoriatic lesions and is correlated with the severity of disease, indicating the important role of GLUT-1 in psoriasis.253,254 Further studies suggest that GLUT-1 is required for the proliferation and stress responses of keratinocytes. Its important role is demonstrated by an animal study, which has shown that specific genetic inhibition of GLUT-1 in keratinocytes can decrease psoriasiform hyperplasia in an IMQ-induced psoriasis-like mouse model. Moreover, topical application of GLUT inhibitor can also alleviate different forms of psoriasis in animal models, indicating that glucose transport might be a promising therapeutic target of psoriasis.255 Lately, our group found that HIF-1 α could promote glycolysis, which also plays a crucial role in psoriasis, by upregulating CD147/Basigin and GLUT1.256 Pyruvate kinase M2 (PKM2), a key rate-limiting enzyme of glycolysis, has a high level of expression in psoriasis. EGF and IL-17 may both contribute to the upregulation of PKM2.257,258 It has been demonstrated that PKM2 is essential for the proliferation of keratinocytes. Conditional PKM2 knockout in keratinocytes could greatly reduce the severity of skin lesions in an IMQ-induced psoriasis-like mouse model.257 Recent studies have found that the effect of PKM2 on keratinocytes is mainly exerted through a complex formed with PKM2, Act1, and TRAF6. Subsequently, the complex regulates the NF-κB transcriptional signaling downstream of IL-17 signaling.258 In addition to acting on keratinocytes, PKM2 can also enhance the differentiation of Th17 cells, a crucial pathogenetic cell in psoriasis, by activating STAT3 or HIF-1α.259,260 All the above evidence suggests that PKM2 might be a potent therapeutic target for psoriasis. As a product of glycolysis, lactate (or lactate acid) modulates immune responses in inflammatory and tumor microenvironments.261 Lactate dehydrogenase (LDH) mediates the production of lactate.262 The lactate is mainly transported into the extracellular space by two symporters, MCT1 and MCT4, with the aid of CD147.263 CD147, an integral transmembrane protein in the immunoglobulin superfamily, can regulate glycolysis associated with MCT1/MCT4 in cancer cells and T cells263,264; CD147 also regulates the MCT1 expression and lactate export.265 Watanabe et al. found that the serum LDH level might be an indicator of treatment preference, as there was a correlation between clinical improvement in patients treated with apremilast but not in those treated with biologics. However, the serum LDH level was not correlated with the severity of cutaneous disease.266 Whether LDH can be a biomarker for psoriasis prediction or efficacy prediction needs more research to explore. Evidence also suggests a significant role of CD147 in the pathogenesis of psoriasis. As early as 2010, our team found elevated CD147 on neutrophils in the peripheral blood of patients with psoriasis, which induced neutrophil chemotaxis267; over the next year, we found that CD147 was a molecular marker of high proliferation and low differentiation of keratinocytes. And CD147 might be a psoriasis susceptibility gene. Our further studies demonstrated that the upregulation of CD147 was stimulated by IL-22 via the activation of STAT3 and that CD147 knockdown could reduce the psoriatic changes, such as the production of cytokines, chemokines, and antimicrobial factors induced by IL-22.268 As discussed above, Th17 cells play a central role in psoriasis pathogenesis, and the energy required for the differentiation of Th17 cells comes from glycolysis. According to a study by Kanekura et al., CD147 plays an essential role in the development of psoriasis through the induction of Th17 cell differentiation,269 expanding the pathologic implication of CD147 in psoriasis.
Metabolites of the TCA cycle
The TCA cycle is a significant pathway for energy metabolism, and the metabolites also play a crucial role in a variety of physiological processes.270 Recent studies have shown the important role of the metabolites of the TCA cycle in the pathogenesis of psoriasis. Specifically, metabolites like lactic acid, pyruvic acid, malic acid, and alpha-ketoneglutaric acid were found to increase in the skin of psoriatic mice, while some other metabolites, such as itaconic acid, were found decreasing.271 However, the sample size of this study was limited, indicating that further research is needed to verify the exact mechanism. On this basis, a study by Li et al. emphasizes the significance of metabolites of the TCA cycle in the pathogenesis and treatment of psoriasis. According to their study, the TCA cycle is disordered in patients with psoriasis, and the relapse of this disease might be related to the deficiency of the TCA cycle function. Among the affected metabolites, fumaric acid and itaconic acid are regarded as potential biomarkers for the treatment and pathogenesis of psoriasis,272 which is in line with previous findings. Fumarate, as an anti-inflammatory factor, has been found to induce the upregulation of HO-1, further impairing IL-23p19 transcription and STAT1 activation to suppress IL-12 and IL-23.273 Clinically, dimethyl fumarate, a fumaric acid ester, is applied to treat moderate-to-severe plaque psoriasis.274 Itaconate, a derivative of the TCA cycle, has proven to inhibit IκBζ production, which relies on ATF3.275 Itaconate is also regarded as an endogenous SDH inhibitor, while SDH enzyme plays a critical role in the activation and function of human T cells.276 Importantly, dimethyl itaconate can markedly alleviate psoriatic-like changes in imiquimod-induced psoriasis-like murine model.275 Taken together, the disorder of the TCA cycle plays an important role in the pathogenesis and recurrence of psoriasis, and the intervention on metabolites of the TCA cycle may be a potential strategy to cure psoriasis. However, there are still few studies focusing on the role and mechanism of the TCA cycle in psoriasis. Thus, further research is needed.
Metabolites of amino acid metabolism
The dysregulation of amino acid metabolism, especially branched-chain amino acid (BCAA) and glutamine metabolism, is also believed to be involved in psoriasis pathogenesis. As early as 2015, Wheelock et al. found that the severity of psoriasis was significantly related to the level of circulating amino acids. Etanercept can also reverse the distinct psoriatic metabotype to a healthy level, indicating that the monitoring of metabolic indicators can help to predict patient response to therapies.277 In 2021, our group found that the level of some amino acids, especially essential amino acids (EAAs) and BCAAs, are significantly altered in patients with psoriasis; to be specific, these amino acids included aspartic acid, glutamate, EAAs, BCAAs (leucine, isoleucine, and valine), ornithine, phosphoserine (Pser), other hydrophobic amino acids such as alanine and proline, and aromatic amino acids (AAAs, e.g., phenylalanine and tyrosine).278 These amino acids are also related to metabolic diseases such as obesity and insulin resistance, both of which are associated with psoriasis. These findings indicate that amino acid metabolism plays a crucial role in the pathogenesis of psoriasis. However, further studies are still needed to elucidate whether elevated EAAs, BCAAs, and glutamate and downregulated glutamine can predict the risk of psoriasis as well as to investigate the underlying mechanism. In addition, glutamine, the most abundant amino acid in the bloodstream, is also regarded as an important substance with altered levels of psoriasis. Glutamine is an important metabolic fuel, which helps cells rapidly proliferate to meet the increased demand for ATP and biosynthetic precursors. It is transported into cells via the transporter, ASCT2/SLC1A5, and then converted to glutamate in the mitochondria through deamination catalyzed by glutaminase (GLS).279 Prior metabolomic analyses have revealed an increased glutamate metabolism in patients with psoriasis, which is positively correlated with the PASI score.277 It is also found that glutamine is important for the differentiation of naïve CD4+ T cells to Th17 cells, and that ASCT2/SLC1A5 is necessary for the production of Th1 and Th17 cells and inflammatory T cell responses.280 In addition, GLS1-mediated glutaminolysis, is also found to be related to psoriasis pathogenesis. With regard to the mechanism, GLS-1 can promote Th17 and γδ T17 cell differentiation by enhancing the acetylation of histone 3 induced by acetyl-CoA in the Il-17a promoter. Furthermore, GLS1-mediated glutaminolysis can also enhance the proliferation of keratinocytes and the release of chemokines.281 This provides a new insight into the link between metabolism and inflammation in psoriasis and indicates that GLS-1 may be a therapeutic target for psoriasis. Interestingly, SLC7A5, also known as LAT1 and being a mediator for the uptake of large neutral amino acids has increased transcriptional levels in psoriatic lesions.282 Cibrian et al. further found increased expression of LAT1 protein in keratinocytes and infiltration of lymphocytes in psoriatic lesions. Thus, targeting LAT1-mediated amino acid uptake may also be a useful strategy to control skin inflammation by blocking the expansion of γδ T cells and IL-17 secretion by CD4 T cells.283
Metabolites of lipid metabolism
Lipid metabolism involves lipid synthesis and degradation. The former mainly involves the synthesis of structural and functional lipids, such as glycolipids, phospholipids, sphingolipids, and cholesterol.284 To date, lipid metabolism has become a major research interest, as lipids play an important role in almost all biological mechanisms, including the formation and maintenance of the skin barrier.285 Studies on lipid metabolism in psoriasis started at the beginning of the 20th century due to the changes in cholesterol levels found in patients with psoriasis.286 Subsequent studies have found abnormal bioactive lipids in psoriatic lesions.287,288 Among them, sphingolipids attracted more attention due to their effect on keratinocyte growth in psoriasis.289 Sphingosine has four derivatives, namely sphingosine-1-phosphate (S1P), ceramide-1-phosphate (C1P), ceramide (Cer), and sphingomyelin (SM). To date, there are controversial views about the role of S1P in psoriasis. Flisiak et al. found a lower serum Cer concentration and significantly higher S1P concentration in patients with psoriasis compared to healthy individuals; however, the S1P level was not related to the severity or duration of psoriasis.290 It is reported that S1P exerts anti-proliferative and anti-inflammatory effects in psoriatic mouse models.291 Further studies showed that elevating S1P by inhibiting S1P lyase could moderate keratinocyte differentiation and reduce cell proliferation, which was followed by amelioration of IMQ-induced psoriasis-like dermatitis.292 Nevertheless, Park et al. pointed out that blocking the production of S1P by a sphingosine kinase 1/2 inhibitor could induce IMQ-induced skin lesions and inflammation through the inhibition of Th17 cell differentiation.293 More importantly, a phase II trial revealed that oral ponesimod, a functional antagonist of S1P, is effective in the treatment of moderate to severe chronic plaque psoriasis.294 It is without doubt that there were some limitations in these studies, such as the short treatment duration and highly selected population. Hence, more evidence is needed to confirm the effect of S1P on psoriasis. Recently, our group found that there was a significantly higher level of C1P and ceramides phosphate (CerP) in patients with psoriasis, with the higher level of CerP suggesting more severe psoriasis. Moreover, C1P was found to promote inflammation in the IMQ-induced psoriasis-like mouse model, and inhibition of C1P production via a ceramide kinase inhibitor could alleviate the psoriasis-like inflammation.295 In addition, using an untargeted lipidomics approach, our team found that substances involved in glycerophospholipid metabolism, such as lysophosphatidic acid (LPA), lysophosphatidylcholine (LysoPC), and phosphatidylinositol (PI), were significantly upregulated in the plasma of patients with psoriasis. This discovery sheds new light on the role of lipids in psoriasis.296 Our subsequent studies found that LPA could mediate the pathogenesis of psoriasis by activating keratinocytes through LPAR5.297,298 In addition to the influence on keratinocytes, lipid metabolism also plays a vital role in immune cells related to psoriasis, such as plasmacytoid dendritic cells, Th17 cells, and macrophages.293,299,300,301,302 On the basis of the above finding, psoriasis is also regarded as an immunometabolic disease.303 In the future, it is worth working hard to validate the predictive value of such lipid metabolites as biomarkers in psoriasis.
In summary, both keratinocytes and immune cells in psoriasis can be characterized by metabolic disruptions. What a pity is there is a lack of comprehensive understanding of the link between immunology and metabolism. Excitingly, nowadays, studies have yielded insightful findings prompting researchers to investigate potential biomarkers, which will offer novel tools for monitoring and managing psoriasis. Moreover, metabolic reprogramming via suppressing of affected metabolic pathways or metabolites and the dietary restoration of metabolic imbalances may be a prominent therapeutic opportunity to achieve long-term management of psoriasis with minimum adverse effects.
Epigenetic regulation
Psoriasis is a chronic and recurrent inflammatory skin disease affected by the complex interplay between genetic and environmental factors,11 especially in the presence of the HLA-C*06:02 risk allele, as well as environmental triggers such as infection, stress, smoking, unhealthy diet, medications, and alcohol consumption.12 In addition to the genetic differences between monozygotic twins, epigenetic mechanisms have also attracted great attention.304,305,306 Epigenetic modification refers to heritable changes in gene function that take place without any changes in the DNA sequence; this includes DNA methylation, histone modifications, non-coding RNAs, and newly-discovered N6-methyladenosine (m6A).307 To date, epigenetic studies have provided new strategies for the treatment of psoriasis.308,309 In this section, we look to present the major epigenetic mechanisms and their roles in the pathogenesis of psoriasis (Fig. 5).
Epigenetic regulations in psoriasis. Environmental triggers such as smoking, medications, diet, alcohol consumption, infection, and stress can alter the expression of genes without changing the DNA sequence. This graph shows the main paradigms of epigenetic modification of psoriasis. Created with BioRender.com
DNA methylation
DNA methylation is a process to transfer a methyl group from S-adenosylmethionine (SAM) to the 5-carbon position of a cytosine ring catalyzed by DNA methyltransferases (DNMTs), which occurs mainly in CpG dinucleotides.310 Research on DNA methylation in psoriasis encompasses two fundamental aspects: genome-wide analysis and the methylation patterns of specific gene loci. Early studies of the epigenetic profile of psoriasis found altered global DNA methylation in psoriatic skin compared to healthy controls.311,312,313,314 Roberson et al. first identified the methylation of 27,578 CpG sites in skin lesions of patients with psoriasis compared with normal controls; among them, 1108 were differentially methylated, and 12 of these sites were mapped for their epidermal function and differentiation. Moreover, the level of methylations can be reversed after one month of anti-TNF-α treatment.311 This observation implies that gene methylation levels may potentially serve as a valuable indicator for monitoring the effectiveness of psoriasis treatment. Zhang et al. further demonstrated that the level of global DNA methylation was elevated in the affected skin and peripheral mononuclear blood cells (PBMCs). They also found a positive correlation between the PASI score and the degree of DNA methylation, rather than the level of PBMC methylation, in the skin lesions of patients with psoriasis.315 This indicates that the methylation levels may potentially function as a valuable indicator for clinical diagnosis. However, when methylation of the long interspersed nuclear element-1 (LINE-1) was assessed, hypomethylation was observed in psoriatic skin, with downregulated expression of genes containing LINE-1.316 LINE-1 is a retrotransposable element accounting for approximately 20% of the human genome and is commonly used as a surrogate for global methylation.317 Nevertheless, the precise mechanism and functional implications of genome-wide methylation in psoriasis remain inadequately elucidated, necessitating further comprehensive investigation.
Zhou et al. observed distinct variations in the methylation levels of these genes using a combination of 262 skin samples and 48 PBMC samples. Notably, genes such as CYP2S1 and S100A8 exhibited such variations.314 CYP2S1 encodes a cytochrome P450 protein that is able to upregulate the activity of cAMP-dependent protein kinase in fibroblasts by catalyzing the metabolism of retinoids.318 It also inhibits KC proliferation and modulates immune responses through cytokines such as IL-8, IL-33, and CXCL-10.319 The S100A family also holds significant sway over the proliferation of KCs and inflammatory response.320,321 The study of specific gene methylation patterns can serve as the foundation for subsequent investigations, to identify potential psoriasis susceptibility biomarkers. In addition, it has been found that there is a certain correlation between differentially methylated genes and typical histopathological abnormalities of psoriasis (e.g., Munro microabscesses, parakeratosis, and neutrophil infiltration).322,323,324 However, most of the current studies on DNA methylation in psoriasis used skin tissue or peripheral blood as samples, and cellular heterogeneity might have interfered with the actual influence of epigenetics on psoriasis.
Some studies have suggested that DNA methylation may be a therapeutic target for psoriasis, as some drugs can modulate DNA methylation, affecting the expression of genes involved in inflammation and immune responses.321,325,326 Wnt inhibitory factor-1 (WIF1) is a molecule that inhibits the Wnt signaling pathway to regulate cell proliferation and differentiation. It is found that abnormal DNA methylation of WIF1 promoter can reduce its expression, resulting in the proliferation of keratinocytes and the production of IL-8. Treatment with a DNA methylation inhibitor, decitabine, can inhibit the development of psoriasis in IMQ mouse model.327 Currently, there are relatively few studies on the treatment of psoriasis that target DNA methylation, but the importance of DNA methylation in psoriasis cannot be ignored due to its implications in the selection of treatments.
Histone modifications
Histones are structural proteins of chromatin, which form the basic unit of chromatin – nucleosomes with DNA. To date, five types of histones have been found, namely Histone 1 (H1), Histone 2A (H2A), Histone 2B (H2B), Histone 3 (H3), and Histone 4 (H4).328 The N-termini of these histones are prone to post-translational modifications (PTM), forming methylation, acetylation, phosphorylation, ubiquitination and other covalent modifications with amino acid residues. In recent years, new types of modifications such as β-hydroxybutyrylation, glycosylation, and lactylation have also been discovered.329,330,331,332 The histone PTMs can influence gene transcription by inducing local structural changes of chromatin, regulating gene transcription, or by indirectly binding to effect proteins or chromatin remodeling complexes. They can also dynamically regulate the genome by affecting the replication and repair of DNA.333 Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are in control of the balance between histone acetylation and deacetylation. Altered expression of the two enzymes has also been found in patients with psoriasis.334 Zhang et al. found that the level of H4 histone acetylation in PBMCs of patients with psoriasis was significantly lower than that of healthy controls. And the acetylation level of patients was negatively correlated with the PASI score.335 All of the above findings suggest that histone acetylation may play a role in psoriasis. However, it has also been found that the activities of HDAC1, HDAC2, and HDAC3 in psoriatic lesions are not significantly different from those in healthy controls,336 which might be related to the different severities of psoriasis in patients included in different groups of the study. The IL-23/IL-17 axis has been identified as a central role in psoriasis. With the advances in research, the histone acetylation of specific genes in this axis has attracted great attention. Xia et al. found that increased histone H3 acetylation of the IL17a promoter could promote Th17 and γδ T17 cell differentiation, which contributed to the immune imbalance and development of psoriasis.281 Using a mouse model, Li et al. found that TNF could inhibit both G9A and CLP, which are methyltransferases of H3K9, by altering the level of phosphorylation of related proteins in keratinocytes, thereby decreasing the level of H3K9 methylation and increasing the level of IL-23.337 Recently, medications targeting histone modifications have become a hot spot of studies. Vorinostat, an HDAC inhibitor, is found to inhibit KC proliferation to induce their differentiation and apoptosis.338 Piperlongumine (PPL) can epigenetically inhibit histone-modifying enzymes, effectively enhancing the interactions of HDAC3 and p65 with IκB, indicating that PPL may be a potential medication in the treatment of psoriasis due to its suppression effect on cell proliferation and inflammation.339
In these studies, the abnormal regulation of histone acetylation in psoriasis has been associated with various critical processes, especially inflammatory responses and the proliferation of keratinocytes. This aberrant regulation can induce chromatin relaxation, subsequently modifying the expression of specific genes. Such alterations play a role in driving the development and progression of psoriasis. Up to now, treatment targeting histone modifications is still at the level of cellular and animal models. It is far away to verify its safety and efficacy. It is undeniable that ongoing research efforts will contribute significantly to enhancing our comprehension of this intricate regulatory network, ultimately providing a novel theoretical foundation for the advancement of therapies and treatments for psoriasis.
Non-coding RNA
With the progress of gene sequencing technology in recent years, the role of non-coding RNA (ncRNA) has attracted great attention as a functional regulatory molecule that mediates a variety of cellular processes, such as chromatin remodeling, transcription, post-transcriptional modification, and signal transduction.340,341,342,343 There are various types of ncRNAs, including microRNA (miRNA), long noncoding RNA (lncRNA), circular RNA (circ RNA), and circular RNA (circRNA).344 With the advance of research, an increasing number of ncRNAs have been found to be associated with the pathophysiology and pathogenesis of psoriasis by regulating the proliferation and differentiation of keratinocytes, secretion of chemokines or cytokines, and activity of T cells.309,345,346
MiRNAs are a form of small non-coding RNAs (consisting of around 22 nucleotides). They inhibit gene expression at the post-transcriptional level by specific complementary binding to their target messenger RNAs. A growing body of evidence suggests that miRNAs play an important regulatory role in metabolism, differentiation, inflammation, immunity, and canceration.347,348,349,350 Altered expression of miRNAs such as miR-210, miR-31, miR-149, miR-125, and miR-155 has been found in skin lesions, peripheral blood mononuclear cells, and plasma of patients with psoriasis.309,351 In this section, we look to summarize the pathogenesis of psoriasis related to miRNAs through the influence on a variety of pathways involving immune regulation and keratinocytes.
MiR-210, a hypoxia-associated miRNA, is significantly upregulated in the skin lesions of patients with psoriasis and IMQ-induced mouse models. It is found that miR-210 can promote Th17 and Th1 cell differentiation while inhibiting Th2 cell differentiation through STAT6 and LYN, leading to immune imbalance and inflammatory reactions.345 MiR-155 is a central regulatory miRNA that is simultaneously involved in the regulation of multiple genes critical for functional epidermal development.352 MiR-155 has been found upregulated in psoriatic skin lesions, LPS-induced keratinocytes, and monocytes353,354,355,356,357 and to further regulate psoriatic processes, including the proliferation of psoriatic mesenchymal (PM) stem cells, the release of cytokines or chemokines of psoriatic keratinocytes, and Th17 inflammatory responses. With regard to the mechanism, miR-155 regulates the production of IL-6 and CXCL8 in keratinocytes through the GATA3/IL-37 regulatory axis.356 The expression of miR-155 is also increased in CD14+ monocytes, which may regulate the proliferation and expression of proinflammatory cytokines as well as the oxidative stress of CD14+ monocytes through the TLR4/MyD88/NF-κB signaling pathway.353 Moreover, miR-155 may also promote the glycolysis of PM by negatively regulating the TP53INP1/p53 signaling pathway, thereby promoting the proliferation of PM.357 MiR-31 has been found upregulated in psoriatic lesions to increase IL-22 expression by targeting periodic tryptophan protein 1 (Pwp1), which is associated with histone modification.358 Wang et al. also found that miR-31 could enhance glutamine metabolism and induce Th17 cell differentiation to participate in the development of psoriasis,359 which is a new finding on immune-metabolic interaction. However, it is also found that miR-31 is decreased in dermal mesenchymal stem cells (DMSCs), which promote T cell activation by inhibiting the proliferation of DMSCs.360 The differences in miR-31 expression in the above studies might have been related to the different cell types studies; thus, the mechanism should still be further explored.
The MiR-125 family can be divided into miR-125a and miR-125b subfamilies.361 Raaby et al. found that the expression of miR-125a was downregulated in skin lesions of patients with psoriasis,362 and Su et al. also found that miR-125a expression was negatively correlated with the surface area of skin lesions, the Psoriasis Area and Severity Index (PASI) score, the use of phototherapy, and the expression of TNF-α, IL-1β, and IL-17 in patients with psoriasis, which might be indicators for psoriasis. Meanwhile, miR-125a can inhibit KC proliferation and promote apoptosis by negatively regulating the IL-23R/JAK2/STAT3 signaling pathway.363 MiR-125b is also found to be downregulated in the skin, plasma, and M5-stimulated KCs in patients with psoriasis, which may affect the proliferation and apoptosis of HaCat cells through the targeted binding to STAT3, BRD4, and SIRT6 and regulate inflammatory responses.364,365,366 Chowdhari et al. found that miR-4516 was downregulated in the skin lesion of patients with psoriasis;367,368 miR-4516 binds to STAT3 to inhibit the expression of Bclxl and Cyclin D1368 and also targets extracellular matrix protein genes to participate in the differentiation and migration of keratinocytes.367 MiR-149 inhibits the production of IL-6, which is induced by TWEAK through the phosphorylation of p38. Furthermore, it is found that high levels of IFN-γ can inhibit the expression of miRNA-149, thereby promoting inflammatory responses in psoriasis.369 Other miRNA expression profiles and corresponding targets in psoriasis are presented in Table 1.
LncRNAs are non-coding RNAs consisting of approximately 200 nucleotides and play an important role in autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, ulcerative colitis, and psoriasis.370,371,372,373 Using RNA sequencing, Tsoi et al. found that more than 40% of novel lncRNAs were differentially expressed in psoriatic lesions. These lncRNAs were found to be co-expressed with immune-related genes and enriched in epidermal differentiation complexes, suggesting that they might be involved in the pathogenesis of psoriasis.374 Through the comparison of skin group between patients with psoriasis and healthy volunteers using microarray technology, Yan et al. found significantly altered expression in 2194 lncRNAs and 1725 mRNAs.373 To be specific, the expression of NORAD was found upregulated in IL-22/LPS-stimulated HaCaT cells, possibly through the negative regulation of miR-26a, thereby upregulating the expression of proteins associated with keratinocyte proliferation (e.g., K6, K16, and K17).375 MSX2P1, which is highly expressed in psoriatic lesions, promotes keratinocyte growth by inhibiting miR-6731-5p and activating S100A7.376 Furthermore, MIR31HG and RP6-65G23.1 can accelerate HaCaT cell proliferation by promoting the cell cycle.377,378 Some lncRNAs, such as MEG3 and LOC285194, are significantly downregulated in psoriatic lesions. MEG3 inhibits keratinocyte proliferation by regulating the expression of miR-21 and caspase-8.379 LOC285194 negatively regulates the expression of miR-616, which in turn leads to GATA3 activation, ultimately reducing keratinocyte viability.380 By comparing patients with psoriasis and individuals with no skin diseases, a study identified 971 differentially expressed lncRNAs and 157 differentially expressed lncRNAs after treatment with adalimumab, highlighting the potential role of lncRNAs in the physiology of psoriasis and patient response to treatment.381 The lncRNA TRAF3IP2-AS1 regulates Act1-meditated IL-17A signaling by inhibiting IRF1, a transcription factor of Act1; this might be the reason why patients with psoriasis who have the Act1 (D10N) variant are unlikely to respond well to IL-17A inhibitors.382
CircRNAs, a special form of ncRNA molecules generated by alternative splicing, are evolutionarily highly conserved.383 It can competitively bind to miRNAs to attenuate the translational repression or degradation of their target gene mRNAs via miRNAs.384 Increasing evidence suggests that circRNAs are involved in psoriasis pathogenesis by regulating cell proliferation and inflammation.385,386,387 Qiao et al. found that hsa_circ_0061012 was significantly upregulated in psoriatic lesions and that it might be a candidate biomarker for psoriasis.388 In addition, circ_0003738 is significantly up-regulated in Treg cells in psoriasis and may reduce the inhibitory function of Tregs through miR-562/IL-17RA and miR-490-5p/IFNGR2, thereby promoting the inflammatory responses in psoriasis.389
In summary, ncRNA-mediated epigenetic regulation is closely related to the development and progression of psoriasis. The ncRNAs detected in blood or urine samples can be used as biomarkers for diagnosis and prognosis. Transport of ncRNAs to skin tissues by modified nucleic acid technology may be a potential alternative treatment for psoriasis. However, high complexity is still a problem in the application of ncRNAs in clinical practice, and the specific effect of each form remains to be further confirmed in more preclinical studies.
m6A methylation
With the development of sequencing technology in recent years, m6A has gradually become a hot spot in epigenetic studies. The m6A modification refers to the methylation of the nitrogen atom at the 6th site of adenine in RNA molecules. It is found that m6A methylation is involved in a variety of biological behaviors of cells, which is a highly specific, dynamic, and reversible biological process that involves three types of enzymes: methylation transfer protein (writer), demethylation protein (eraser) and recognition protein (reader).390,391 Using RNA sequencing, Wang et al. found that transcripts of the psoriatic skin sample had the fewest m6A peaks and the lowest m6A peak density. Besides, the greatest differences in m6A methylation were observed in the comparison between psoriatic skin and skin of healthy individuals or between psoriatic skin and unaffected skin of patients with psoriasis. Moreover, the upregulation of gene expression was often accompanied by the upregulation of m6A methylation, suggesting a regulatory role of m6A in psoriasis.392 Oerum et al. found that hsa_circ_0004287 was upregulated in PBMCs of patients with psoriasis. This upregulation appears to be associated with the impact of has_circ_0004287 on reducing the stability of its host gene metastasis associated lung adenocarcinoma transcript 1 (MALAT1) by competitively binding to IGF2BP3 in an m6A-dependent manner, which subsequently leads to the phosphorylation of p38/mitogen-activated protein kinase and the initiation of macrophage-mediated inflammation.346 Xia et al. also found that AGAP2-AS1 could be upregulated in the skin of patients with psoriasis through the modification of m6A methylation, thereby promoting keratinocyte proliferation through the miR-424-5p/AKT/mTOR axis.393 Lately, Xiao et al. concluded that METTL3-mediated m6A methylation equilibrated γδ T1 and γδ T17 cells to participate in the pathogenesis of psoriasis.394 However, there is still a lack of studies on m6A, suggesting the need for more experiments to demonstrate the role of m6A in psoriasis.
Comorbidities of psoriasis and their molecular mechanistic cascades
The incidence of comorbidities in patients with psoriasis was first reported in 1897, indicating the early awareness of the coexistence of psoriasis and other diseases as well as the early exploration of common pathogenic mechanisms.395 Subsequent clinical and epidemiological studies have shown that patients with psoriasis may have multiple comorbidities, including metabolic syndrome (e.g., diabetes, hypertension, dyslipidemia, and obesity), cardiovascular disease, depression, and psoriatic arthritis.396,397,398 Some clinical studies suggested a wider variety of comorbidities of psoriasis, including schizophrenia, asthma, inflammatory bowel disease (IBD), Crohn’s disease, chronic kidney disease, and Grave’s disease.399 Furthermore, an increasing number of studies have shown that psoriasis is associated with an increased risk of cancer.400,401 The presence of psoriasis comorbidities can also adversely affect the outcomes, quality of life, life expectancy, as well as psychosocial and economic status of patients. A better understanding of the involved signaling pathways and physiological mechanisms can be helpful for treating psoriasis and its comorbidities as early as possible (Fig. 6).
Mechanistic models of psoriasis comorbidities. Chronic inflammatory processes directly lead to psoriatic arthritis, and they contribute to other comorbidities when coexisting with other factors. Driven by genetic factors and increased cytokines, diabetes, and IBD are likely to develop in psoriasis. Dyslipidemia, together with abnormal cytokine levels, can lead to obesity and coronary heart diseases. With a hyperactive HPA axis, psoriatic cytokines can further lead to depression. Created with BioRender.com
Common genetic background, increased oxidative stress, overlapping chronic inflammatory processes, abnormal immune regulation mechanisms, and the brain-skin axis may be the basis of multiple comorbidities of psoriasis. Among them, the abnormal immunopathophysiological events play a central role.
Prior GWAS have identified several shared genetic loci (including PSORS2, PSORS3, and PSORS4) and mutual genes (including CDKAL1, ST6GAL1, PTPN22, and JAZF1) that link psoriasis and the susceptibility to diabetes.402,403 Moreover, the increased key cytokines in psoriasis, such as IL-23, IL-17, IL-1β, IFN-γ, and TNF-α can damage islet β cells in a separate and synergistic manner. For instance, IL-1β can induce insulin resistance by activating the p38MAPK pathway,404 and TNF-α mediates insulin resistance by inhibiting the activity of insulin receptors to contribute to the development of diabetes.405 These may be the potential molecular mechanisms of the comorbidity of diabetes in patients with psoriasis. Driven by genetic factors and increased cytokines, IBD can also appear as a comorbidity of psoriasis.406,407
Although current epidemiological data from cross-sectional and observational studies have suggested a high risk of hypertension in patients with psoriasis, the exact pathophysiological mechanism is still unclear. Possible mechanisms include the release of keratinocyte-derived vasoactive peptides such as endothelin-1.408 Excessive endothelin-1 release by hyperplastic keratinocytes of psoriasis can lead to vascular inflammation and endothelial dysfunction via promoting cytokine and ROS production, which has been identified as a primary feature of hypertension pathophysiology.409,410
Dyslipidemia is frequently observed in patients with psoriasis; in such patients, decreased levels of high-density lipoprotein (HDL) or increased levels of low-density lipoprotein (LDL), especially oxidative LDL, have been found.411 The increased oxidized LDL in psoriasis patients is engulfed by macrophages and forms arterial plaque, leading to cardiovascular diseases such as atherosclerosis and coronary artery disease (CHD).412 Moreover, the released IL-17, IL-22, IL-6, and IL-21 in psoriasis can cause plaque instability and hemorrhage.413 In addition to CHD, dyslipidemia is also an independent risk factor for comorbidities of psoriasis, such as obesity.414 It has been found that a variety of psoriasis-related cytokines, such as TNF-α, IL-1β, and IL-6, can lead to obesity by regulating triglyceride metabolism and adipocyte differentiation.415
Among all the comorbidities, depression is the most common and relevant neurological comorbidity in patients with psoriasis.416 Numerous studies have shown that mental stress is an essential contributor to psoriasis, and the deterioration of psoriasis can increase the risk of depression and anxiety.417 The brain-skin axis is believed to play an essential role in this process.418,419 It is revealed that cytokines such as IL-1β, TNF-α, and IL-6, which are increased in psoriasis, can lead to the increase in cortisol by downregulating the negative feedback loop of the HPA system, resulting in hyperactivity of the HPA system and ensuing depression.420
Current progress in targeted therapy for psoriasis
Overview of therapeutic options
Psoriasis has a high recurrence rate despite a variety of treatment options.12 In clinical settings, the aims of treatment for psoriasis include removing skin lesions, reducing itching and other distressing symptoms, and improving patients’ quality of life. It is also necessary to reduce the frequency of psoriasis recurrence and its complications and control adverse reactions during the treatment.421 The selection of treatment approaches is based on the accurate assessment of the severity of this disease, which is usually performed using the PASI. The PASI is rated from aspects of the location, area, scaling, infiltration, and erythema of the lesions, with a total score of 0–72.422 According to the consensus, mild psoriasis was defined as ‘PASI ≤ 10’, and for moderate-to-severe psoriasis, ‘PASI > 10’.423 Apart from PASI, Physician Global Assessment (PGA), Nail Psoriasis Severity Index (NAPSI), Pustular Psoriasis Physician Global Assessment (GPPGA), and American College of Rheumatology criteria (ACR) are also used to assess the severity of different types of psoriasis.424,425
Topical corticosteroids, calcipotriol, or their combination are the primary treatment methods for mild psoriasis.12 Topical corticosteroids exert anti-inflammatory, antiproliferative, and local vasoconstrictive effects by downregulating genes that encode pro-inflammatory cytokines.11 A study found that 40–75% of patients with psoriasis achieved PASI 75 response after using topical corticosteroids.426 However, potent corticosteroids cannot be used long-term due to their adverse effects of subcutaneous fat atrophy and adrenal suppression.427 Calcipotriol is a vitamin D3 derivative that can inhibit epidermal hyperplasia and induce normal epidermal differentiation in psoriasis.428 It has been found that the combined formulation of these two medications, calcipotriol/betamethasone, has better efficacy and remission rate than the monotherapy with either medication, with less irritation and fewer adverse reactions of the skin.429,430
Other topical medications for psoriasis include topical calcineurin inhibitors, topical keratolytic agents, and coal tar. Topical calcineurin inhibitors exert a therapeutic effect on psoriasis mainly by acting on calcineurin targets, thereby inhibiting T cell activation. Due to their advantage of not causing facial lipoatrophy, calcineurin inhibitors are often used on the face.431 Topical keratolytic agents mainly include tazarotene, which can inhibit keratinocytes, thereby breaking down the thick flakes of psoriatic plaques.11 At present, coal tar is rarely used due to its mutagenicity.
Oral medications, such as methotrexate, cyclosporine, and retinoids, are usually used for moderate-to-severe psoriasis that cannot be controlled by topical drugs.423 Methotrexate was approved for the treatment of psoriasis more than 50 years ago by the US Food and Drug Administration (FDA). As the most commonly used treatment option before the introduction of biologics, methotrexate has a certain effect on all types of psoriasis. Despite its therapeutic effect, methotrexate has unignorable adverse reactions such as hepatotoxicity and severe gastrointestinal reactions;432 therefore, folic acid is often recommended to reduce the side effects during the treatment.11 Cyclosporine is also used to treat different types of severe psoriasis. Flytstrom et al. found that cyclosporine is more effective than methotrexate in the treatment of plaque psoriasis. Still, it is also associated with more severe renal toxicity and other adverse reactions such as gastrointestinal discomforts and hirsutism, which might be why it is limited in clinical practice.433 Unlike methotrexate and cyclosporine, acitretin, a systemic synthetic retinoid, is usually used to treat erythrodermic psoriasis and pustular psoriasis. However, acitretin cannot be used by pregnant individuals or planning for pregnancy because of its teratogenic effect; other adverse effects include dry skin, dyslipidemia, and elevation of transaminases.11,434
Phototherapy is also an important treatment option for moderate to severe psoriasis. There are many types of phototherapies, including psoralen and UV-A (PUVA) (320–400 nm), wide-band ultraviolet B (UVB) (290–320 nm), and narrow-band ultraviolet B (NB-UVB) (311 nm).435 NB-UVB has been used as a first-line phototherapy for plaque psoriasis for its better efficacy, longer remission time, and fewer adverse reactions.435 It is also found that the combination of acitretin and NV-UVB can achieve better efficacy with fewer adverse reactions in the treatment of plaque psoriasis.436
With a deeper understanding of the pathogenesis of psoriasis in the past 20 years, biologics have been gradually used for targeted immunotherapy with promising efficacy and fewer side effects, attracting great attention. Thus, we hereby summarize the targeted therapies for psoriasis (Table 2).
TNFα inhibitors
TNF-α inhibitors are the first biological agents approved for the treatment of moderate-to-severe psoriasis and psoriatic arthritis. At present, three main biologics, i.e., etanercept, infliximab, and adalimumab, are used to treat psoriasis by targeting TNFα to prevent TNF-mediated cellular responses and modulate biological responses that are controlled by additional downstream molecules induced or regulated by TNFs.
Etanercept is a recombinant TNFα receptor fusion protein that can competitively bind to the TNF-α receptor p75 Fc on the cell surface to inhibit TNF-mediated cellular responses.437 It is the first TNFα inhibitor approved by the FDA for the treatment of plaque psoriasis and psoriatic arthritis.438 Prior clinical studies have found that etanercept can significantly relieve plaque psoriasis and psoriatic arthritis; to be specific, this medication helped achieve a PASI response rate of 47–49% at week 12 of treatment with etanercept and a PASI 75 response rate of 59% at week 24 of treatment at a dose of 50 mg twice weekly;439,440 moreover, after 12 weeks of treatment with etanercept for psoriatic arthritis, the ACR 20 reached 66.4–73% with well tolerance.441,442
Infliximab is an IgG1 mouse-derived monoclonal antibody specific for TNFα,443 which was approved by the FDA for the treatment of psoriatic arthritis and plaque psoriasis in 2005 and 2006, respectively.435 Infliximab has prominent effects in treating plaque psoriasis or psoriatic arthritis;443 it is reported that infliximab achieved a PASI 75 response rate of 80–88% at week 10 and helped to maintain a PASI 75 response rate of 72–82% at week 24 when infliximab was used at 5 mg/kg in weeks 0, 2, and 4.444,445 Compared to conventional treatment with methotrexate, infliximab has its advantages in treating plaque psoriasis.446 A prospective study showed that, compared with etanercept, infliximab exerted effects significantly faster before 24 weeks of treatment, but there were no significant differences in efficacy between the two medications at week 48.447 However, there are still some patients with psoriasis experiencing elevated liver enzymes and infusion reactions after using infliximab.443,444,445,446
Adalimumab, an IgG1 monoclonal antibody, has a similar mechanism of action to infliximab. Adalimumab can be used for different types of psoriasis and often has a prolonged effect. In a phase III study from REVEAL, 71% of the patients with plaque psoriasis achieved PASI 75 at week 16 after receiving treatment with adalimumab, while only 7% of the patients receiving placebo achieved PASI 75.448 Importantly, adalimumab can also be used in patients with poor response to etanercept (i.e., patients who did not reach PASI 50); according to a study, the PASI 75 response rates of such a patient group were 27%, 36%, and 54% at week 12, 24, and 48, respectively.449 Two other clinical trials showed an ACR 20 response rates of 65% and 52% after 12 weeks of treatment with adalimumab among patients with psoriatic arthritis.450,451 Of note, it was found that adalimumab was effective on nail psoriasis, with a NAPSI 75 response rate of 46.6% at week 26, compared to only 3.4% in the placebo group.425 According to multiple clinical trials, common adverse reactions of adalimumab include upper respiratory tract infection, rhinitis, and pain at the injection site.425,448,449,450,451
TNF-α inhibitors, the oldest biologics used to treat psoriasis, have been demonstrated to play an important role in different types of psoriasis. However, it is important to note that some patients with psoriasis developed worsening of psoriasis symptoms or new paradoxical psoriasis after the use of TNF-α inhibitors, using TNF-α inhibitors, which were called paradoxical reactions.452 This phenomenon appears to be associated with uncontrollable increased type 1 interferon cytokines, heightened number of Th-1 and Th17 cells, as well as individual genetic susceptibilities.80,453 If paradoxical reactions occur, TNF-α inhibitors will discontinue and change to biologics acting on other pathways, such as ustekinumab, an IL-12/23 antagonist.452
IL-17 inhibitors
IL-17 is a key mediator in the development of psoriasis, and IL-17 inhibitors can treat psoriasis by binding to its ligands or receptors to block the IL-17 pathway.30 To date, three biologic agents targeting the IL-17 cytokine pathway have been approved to treat psoriasis by the FDA, and they include two monoclonal antibodies against interleukin-17A with high affinity, secukinumab and ixekizumab.
Secukinumab is the first monoclonal antibody among IL-17 inhibitors, and it was approved by the FDA in 2015 for the treatment of psoriasis. A randomized, double-blind clinical study showed that secukinumab could reverse the histopathology of plaque psoriasis by reducing IL-17A production, leading to plaque resolution.454 Two key clinical studies, ERASURE and FIXTURE, found that 77.1% and 81.6% of the patients given 300 mg of secukinumab and 67.0% and 71.6% of the patients given 150 mg of secukinumab achieved PASI 75 at week 12; notably, both doses of secukinumab showed better clinical effect than etanercept at a dose of 50 mg.44 In addition to being effective on plaque psoriasis, secukinumab has also proven to be effective in nail psoriasis and psoriatic arthritis. A study showed that 54.0% and 35.0% of the patients with psoriatic arthritis treated with 300 mg of secukinumab achieved ACR 20 and ACR 50 at week 12, respectively. Furthermore, secukinumab is found to have higher treatment retention than adalimumab in the treatment of psoriatic arthritis.424 Secukinumab is also effective in the treatment of nail psoriasis, which is demonstrated by a mean NAPSI improvement of −45.3% at a dose of 300 mg at week 16 and −63.2% at the same dose at week 32, respectively.424 With a similar mechanism to secukinumab, ixekizumab has similar efficacy in the treatment of psoriasis. A phase III trial showed that at least 87.0% of the patients with moderate-to-severe plaque psoriasis achieved a PASI 75 response after receiving continuous treatment of ixekizumab; it is also demonstrated that ixekizumab is superior to etanercept in clinical efficacy.455 However, secukinumab and ixekizumab are associated with adverse events such as infection, headache, neutropenia, and inflammatory bowel disease.44,455,456
In addition to two IL-17A inhibitors, brodalumab, an antibody directly against IL-17RA, has been used in treating psoriasis.30 Two phase III studies (AMAGINE-2 and AMAGINE-3) showed that, after the use of brodalumab via subcutaneous injection at 210 mg once every other week, the PASI 75 response rates were 85.0% and 86.0% at week 12 of treatment.457 In addition to plaque psoriasis, brodalumab is also effective in the treatment of articular psoriasis.458,459
Apart from the three types of IL-17 antibodies approved by the FDA, a new medication, bimekizumab, has already been approved in Europe as of August 2021 and in Japan as of January 2022, is waiting for approval.460,461,462 It is a monoclonal IgG1 antibody that specifically inhibits IL-17F and IL-17A.30 In a multicenter, double-blind, placebo-controlled, randomized withdrawal phase III trial, 91% of the patients with plaque psoriasis achieved PASI 90 response at 16 weeks after the start of bimekizumab administration at 320 mg once every 4 weeks and 89.8% of the patients achieved an Investigator Global Assessment (IGA) score of 0 or 1, indicating that the bimekizumab had faster and stronger effect compared the IL-17 and IL-23 inhibitors used in prior studies, making it a promising new IL-17 inhibitor.460 Bimekizumab is also associated with an excellent response rate in this study, with the relief of skin and joint symptoms lasting through 56 weeks.46,460,463 A recent study has revealed that blimekizumab and adalimumab have similar effects in the treatment of psoriatic arthritis and plaque psoriasis and that bimekizumab is associated with better skin clearance than secukinumab.464 However, blimekizumab is also associated with a higher risk of oral candidiasis. Thus, further studies are needed to weigh its therapeutic values against safety issues.464,465
IL-12/IL-23p40 inhibitors
Ustekinumab was approved by the FDA for the treatment of psoriasis in 2009 and is currently the only IL-12/IL-23p40 inhibitor for this disease.466 It is a monoclonal antibody that specifically binds to the shared p40 subunit of IL-12 and IL-23, blocking the signaling pathways that are activated by normal ligand-receptor binding. In two phase III clinical trials, PHOENIX 1 and PHOENIX 2, the PASI 75 response rates were 66.7% and 67.1% at 12 weeks after the use of ustekinumab at 45 mg and 66.4% and 75.7% at 12 weeks after the use of ustekinumab at 90 mg (both doses were administered at week 0, week 4, and once every 12 weeks thereafter). Moreover, the maintenance of effect of ustekinumab is incredibly long, with a substantial response rate even in the third year after the start of treatment.467,468 Some studies showed that, after 12 weeks of standard treatment at a therapeutic dose for psoriasis, ustekinumab had a higher response rate than etanercept but a lower response rate than brodalumab.47,457 In addition to treating plaque psoriasis, ustekinumab is highly effective for psoriatic arthritis. According to a study, a dose of 90 mg of ustekinumab achieved an ACR 20 response rate of 49.5% after 24 weeks of treatment, compared to 22.8% of the placebo group.469 The strength and duration of the effect of ustekinumab in the treatment of psoriatic arthritis are similar to those of IL-17 inhibitors, such as secukinumab.470,471 Ustekinumab is also effective in treating psoriatic nail diseases, which is demonstrated by a significantly improved NAPSI after 24 weeks of treatment.472 Common adverse reactions to ustekinumab include upper respiratory tract infection, nasopharyngitis, neutropenia, and headache, and it has been found that ustekinumab has fewer adverse effects than TNFα inhibitors.473 At the same time, when paradoxical reactions occur in psoriasis treated with TNF-α inhibitors, switching to ustekinumab may be considered to treat psoriasis and the symptoms of paradoxical reactions that occur.474
IL-23 inhibitors
Monoclonal antibodies that specifically inhibit IL-23 can directly reduce the production of psoriasis-related cytokines. At present, IL-23 antagonists mainly include guselkumab, tildrakizumab, risankizumab, and mirikizumab. Guselkumab is the first IL-23 inhibitor approved by the FDA in 2017 for the treatment of moderate to severe plaque psoriasis,475 and its clinical effect has been demonstrated in different types of psoriasis.476,477,478 VOYAGE 1/2 is the first phase III clinical trial to investigate guselkumab in the treatment of patients with psoriasis; in this study, patients were randomized to receiving placebo, adalimumab, or guselkumab at 100 mg in week 0, week 4, and once every 8 weeks thereafter. The results showed that 85% of the patients using guselkumab achieved an IGA score of 0 or 1 at week 16, and 73% achieved PASI 90 response, which was significantly superior to placebo and adalimumab; however, guselkumab did not show any significant difference in the incidence of adverse reactions from other treatment options.45,479 Tildrakizumab, a humanized monoclonal antibody that can significantly improve plaque psoriatic lesions, has demonstrated by stable efficacy and good tolerability. It also has less effect on risk factors for cardiometabolic diseases, indicating a better safety profile.480,481,482 Moreover, cost-effectiveness analyses of biologics have shown that tildrakizumab is one of the most cost-effective first-line treatment options for moderate to severe psoriasis.483,484 Risankizumab, a humanized IgG1 monoclonal antibody directly against the p19 subunit of IL-23,485 has also demonstrated a better therapeutic effect compared to ustekinumab and adalimumab.486,487 It seems that risankizumab has better efficacy and lower risk compared to other biologics acting on IL-23 p19, IL-12/IL-23 p40 and IL-17.488
IL-36/IL-1 inhibitors
As shown above, the IL-36/IL-1 axis plays an important role in psoriasis, especially in GPP. To date, two humanized IgG monoclonal antibodies targeting the IL-36 receptor (IL-36R), spesolimab and imsidolimab, have demonstrated great efficacy in international clinical trials in patients with GPP.110,489 Spesolimab is a novel humanized selective antibody that blocks the IL-36R and the first medication under development to specifically target the IL-36 pathway for the treatment of acute GPP.490 In a phase I clinical trial on spesolimab, all 7 patients who received a single dose of spesolimab (10 mg/kg) intravenously showed rapid lesion improvement within 4 weeks.491 On this basis, the therapeutic value of spesolimab in GPP was further confirmed by the phase II Effisayil-1 study, which showed that 84.4% of the 35 patients with moderate to severe acute GPP had no visible pustules on the skin after 12 weeks of treatment and that 81.3% achieved clearance or complete clearance of skin symptoms.492 Imsidolimab also demonstrated significant efficacy in the phase II clinical trial GALLOP and has now entered phase III clinical trials.110 In addition to IL-36R, other parts of the IL-1/IL-36 axis are also the focus of studies. Anti-IL-1 agents such as anakinra, canakinumab, and gevokizumab have demonstrated good efficacy in psoriasis. Anakinra is a recombinant IL-1 receptor antagonist (IL-1Ra) that inhibits IL-1α and IL-β, and it has shown good effect in treating pustular psoriasis and deficiency in IL-1 receptor antagonist gene variants.493,494 Canakinumab is also an anti-IL-β antibody with good effects in GPP.495 Gevokizumab is another novel IL-1β antagonist, the effect of which has been preliminarily shown in a study, where two patients with GPP had 79% and 65% improvement in GPP score after 4 weeks of treatment.496 Therefore, IL-1 inhibitors may be effective in the treatment of psoriasis, especially GPP. But large-scale, prospective, randomized clinical trials are needed to confirm the efficacy and safety of these medications in the treatment of acute GPP. Moreover, it was found that IL-8 was significantly decreased in patients with refractory psoriatic arthritis treated with exogenous IL-36Ra, suggesting that the IL-36 axis is among the inflammatory pathways that are most relevant to refractory psoriatic arthritis.497 However, there is no clinical data showing the clinical efficacy of IL-36R-targeted therapies in psoriatic arthritis.
JAK inhibitors
JAK inhibitors are a novel class of immunosuppressive agents that inhibit gene transcription of pro-inflammatory cytokines by blocking the JAK/STAT-mediated intracellular signaling pathways. Tofacitinib is a first-generation JAK inhibitor that mainly targets JAK3, JAK2, and JAK1 and blocks the JAK signal transduction pathway by binding to the ATP-binding site in the JAK protein, thereby relieving psoriatic arthritis. A Phase III study showed that 54% of the patients using tofacitinib at 5 mg twice daily and 63% of the patients using tofacitinib at 10 mg twice daily achieved ACR 20 response at month 3.450 Furthermore, it was approved for the treatment of psoriatic arthritis by FDA.498,499 Despite demonstrating superiority over placebo in treating moderate-to-severe plaque psoriasis, tofacitinib is not approved by the FDA for this indication due to concerns regarding its clinical efficacy and long-term safety.500 Upadacitinib, an oral JAK1 inhibitor approved in 2022 for the treatment of psoriatic arthritis, has also demonstrated good efficacy and safety profile in clinical trials.451,501 However, at present, no clinical trials have been designed to evaluate its efficacy and safety in psoriasis. In addition, baricitinib mainly acts on JAK1 and JAK3 and downregulates cytokines such as interferon-γ, IL-12, IL-17, and IL-23,502,503 and ruxolitinib acts on JAK1 and JAK2, thereby inhibiting the phosphorylation of STAT3 and promoting Th17 cell apoptosis504; both of them are first-generation JAK inhibitors and are effective for the treatment of psoriasis. However, all the first-generation JAK inhibitors inhibit the kinase portion of several JAK proteins, increasing the risk of adverse effects such as infection, hemoglobin reduction, thrombocytopenia, gastrointestinal perforation, and nausea.505,506 Second-generation JAK inhibitors can selectively inhibit individual JAK proteins without affecting other cytokines; for example, deucravacitinib, PF06826647, and brepocitinib selectively act on TYK2.507,508,509,510 Recently, a randomized, double-blind, placebo-controlled phase III clinical trial showed that the PASI 75 response rate reached 58.4% after 16 weeks of treatment with oral deucravacitinib at 6 mg daily, as compared to 12.7% of patients receiving placebo and 35.1% of patients receiving apremilast, with an incidence of adverse reactions similar to that of apremilast.507 Deucravacitinib was approved for the treatment of plaque psoriasis by the FDA in 2022. Although most selective JAK inhibitors are still under investigation and yet to be approved for marketing, they are regarded as promising medications due to their better safety profile.
PDE4 inhibitors
Phosphodiesterase 4 (PDE4) can hydrolyze cyclic adenosine monophosphate (cAMP) and plays an important role in many biological processes. Owing to its broad anti-inflammatory activities, it has been investigated that PDE4 inhibitors can be applied for treating various skin disorders or rheumatic diseases, like psoriasis, PsA, and AD.511 Apremilast, an orally administered PDE4 inhibitor, was approved in the USA in 2014 for adult patients with active psoriatic arthritis or patients with moderate-to-severe plaque psoriasis who were candidates for phototherapy or systemic therapy. Since a PALACE 1 study investigated that a dose of 30 mg twice a day of apremilast achieved an ACR 20 response rate of 40.0% at week 16, compared to 19.0% of the placebo group,512 another phase III clinical trial revealed its efficacy in moderate to severe plaque psoriasis. It is shown that the PASI 75 response rates were 33.1% at week 16 after the use of apremilast at 30 mg twice a day, as compared to 5.3% of patients receiving placebo.513 However, the application of PDE4 inhibitors for the treatment of psoriasis has been hampered by their side effects, such as emesis. After great efforts, based on the DERMIS-1 and DERMIS-2 randomized clinical trials, in 2022, the FDA approved roflumilast as a cream to treat plaque psoriasis, including in skin folds, in patients aged 12 years and older.514 The clinical trials showed that 37.5–42.4% of the patients with plaque psoriasis after 8 weeks of treatment achieved Investigator Global Assessment (IGA) success (clear or almost clear status plus ≥ 2-grade improvement from baseline [score range, 0–4]). Further research is warranted to evaluate the comparative efficacy with other active treatments and to ascertain the long-term effectiveness and safety.
RorγT antagonists
RORγt, mainly expressed in lymphocytes, can regulate the differentiation of CD4+ T cells into Th17 cells, which is closely related to psoriasis. Thus, RORγT inhibition might be an effective strategy for the treatment of psoriasis. VTP-43742 is an oral RORγT inhibitor; in a phase IIa study, a reduction by 29% and 23% in PASI was observed at 4 weeks of VTP-43742 use at 700 mg and 350 mg.515 Other oral RORγT inhibitors under investigation include JTE-451 and ABBV-157, and RORγT inhibitors are regarded as potential candidates for the treatment of psoriasis.516
IL-22 inhibitors
IL-22 may be a new target for the treatment of psoriasis. However, a current clinical study (NCT01010542) on ILV-095, a medication targeting IL-22, was terminated prematurely because the primary efficacy endpoints could not be met. In this regard, we analyzed the reasons for the failure of clinical therapies targeting IL-22. This may indicate that IL-22 plays a protective effect against psoriasis. It has previously been reported that IL-22 can reduce nonalcoholic steatohepatitis by blocking hepatocyte oxidative stress.517 Since elevated ROS in keratinocytes can lead to keratinocyte proliferation and inflammation,518 Whether IL-22 may inhibit oxidative stress in keratinocytes are remaining to be clarified. Besides, it is reported that IL-22 helps maintain the homeostasis of the host immune system and induces immune tolerance by preventing colonization by pathogenic microorganisms.519 Considering the dysregulation of gut microbiota plays an important role in psoriasis,520 we hypothesize that the dysfunction of gut microbial homeostasis may be another reason for the failure of targeting IL-22.
IFN inhibitors
Type I IFN may play a role in the pathogenesis of psoriasis;521 However, according to studies, an IFN-α agonist, MEDI-545, has demonstrated no treatment for plaque psoriasis. This may be related to MEDI-545, which targets IFN-α only. However, besides IFN-α, other types of Type I IFN, like IFN-β, can also participate in the development of psoriasis by promoting Th17 cell activation. Moreover, this could also reflect from the profile that IFN-α and even Type I IFN may not play a major role in psoriasis.
Other clinical research progress
Mesenchymal stem cells (MSCs) are adult pluripotent progenitor cells with low immunogenicity, great self-renewal ability, and strong immunomodulatory properties.522,523 Thus, they are considered a treatment strategy for a variety of autoimmune diseases.524,525 It has been found that MSCs exhibited abnormal apoptosis, proliferation, differentiation, and cytokine secretion in patients with psoriasis.526,527 Furthermore, MSCs can activate the PI3K/AKT signaling pathway to stimulate the proliferation, differentiation, and migration of keratinocytes.528 All the above findings indicated the role of MSCs in the pathogenesis of psoriasis. By co-culturing MSCs of healthy donors with MSCs from patients with psoriasis, Campanatiet et al. found that inflammatory factors such as IL-17A and TNF-α were significantly reduced, which supported the therapeutic value of MSCs.529 In a phase I/IIa, single-arm study on human umbilical cord-derived MSCs (UMSCs), 47.1% of the 17 patients with psoriasis had at least 40% improvement in the PASI score during the treatment with UMSC infusion and through 6 months of follow-up, with a significant increase in Tregs and CD4+ memory T cells and a significant decrease in Th17 and CD4+ naive T cells in the peripheral blood after UMSC transplantation.530 The efficacy of MSCs in psoriasis may be superior to conventional treatments; however, because no large-scale clinical trials on this approach have been conducted, the type, mode, dose, and frequency of MSCs infusion are still under exploration.
The aryl hydrocarbon receptor (AhR) is a transcription factor expressed in skin cells such as keratinocytes, mast cells, and melanocytes.531,532,533 AhR signaling has been shown to regulate Th17 and Th22 cell differentiation, as well as the expression of IL-17 and IL-22,534,535 which have been found to increase in the psoriatic skin in AhR-deficient mice.536 Tapinarof is a topical aryl hydrocarbon receptor modulator separated from a variety of symbiotic bacterial metabolites. It is the first topical cream targeting AhR for psoriasis approved by the FDA.537 In a phase IIa, multicenter, open-label trial, tapinarof showed a certain effect in patients with plaque psoriasis. A study showed that, among 21 patients who received tapinarof cream 1% on a daily basis for 29 days, 18 patients had improved PASI scores, and 7 patients reached PASI 75 response.538 Later, two phase III multicenter, randomized, double-blind, vehicle-controlled trials were conducted on the efficacy and safety of tapinarof; in PSOARING 1, 35.4% of patients using tapinarof (vs. 6.0% of patients who received vehicle cream) daily for 12 weeks achieved the primary endpoint, and in PSOARING 2, the percentage was 40.2% (vs. 6.3% of patients who received vehicle cream).539 In addition, the incidence of adverse reactions, such as burning, stinging, and itching, was low in both trials. Benvitimod cream 1%, a preparation developed in China, has a similar mechanism of action to tapinarof cream 1%; it was approved in China in 2019 for topical use to treat mild-to-moderate plaque psoriasis and is usually used twice daily.540,541
S1P, acting on S1P receptor (S1PR) 1–5, has attracted widespread interest due to its inhibition effect on epidermal cell growth, induction of keratinocyte differentiation, and antiproliferative and anti-inflammatory effects in mouse models of psoriasis.292,542 Ponesimod, a selective S1PR1 agonist for oral use, demonstrated good efficacy in a phase II clinical trial, where 46.0% of patients on ponesimod at 20 mg once daily achieved PASI 75 response at week 16, 48.1% of patients on ponesimod at 40 mg once daily achieved this goal, and the percentage was only 13.4% in patients on placebo.294 The mechanism of S1PR agonist against psoriasis might be related to the reduction of white blood cell count, regional lymph node weight, IL-23 mRNA levels, and CD3+ T cell percentage.543 Known adverse reactions to ponesimod include dyspnea, elevation of liver enzymes, and dizziness; however, as ponesimod has not been intensely studied, its serious side effects still need to be investigated in further studies.294
While traditional therapy and biological agents targeting various inflammatory pathways have successfully treated psoriasis, certain patients still do not respond well. Various clinical studies are underway (Table 3). However, the efficacy is not sure due to the limited sample size and inadequate study duration of the trials. It is necessary to invest more in promising clinical trials. In Table 4, we discussed the advantages and limitations of all therapies available for psoriasis. And it is crucial to keep finding more effective ways to treat psoriasis. Recent studies have shown that metabolism plays an important role in psoriasis. It may be a promising method to focus on targeting psoriasis metabolism and specific metabolic mechanisms in the future. Moreover, an increasing number of research have shown potential connections between psoriasis and gut microbiota. Patients with psoriasis have reduced beneficial microbes in the gut, and gut microbes may cause skin disease by affecting immune balance. There is still insufficient research on the relationship between gut microbiota and psoriasis. Supplementing with probiotics and fecal transplantation also may be promising treatments for psoriasis. More clinical trials are expected to explore their efficiency in the treatment of psoriasis.
The recurrence of psoriasis
The aforementioned biologics have brought great benefits and new hope to patients with psoriasis. However, practical data suggest that psoriatic lesions often recur at the same sites after the discontinuation of the biologics,544 making disease recurrence one of the greatest challenges in the treatment of psoriasis.
A variety of factors, including skin resident memory T (TRM) cells, memory-like γδT cells, and keratinocytes with inflammatory memory, are all considered possible mechanisms for the recurrence of psoriasis (Fig. 7). Among them, TRM cells are recognized as the major driver of psoriasis relapse.545 It is reported that effectors including CD69, integrins (CD49a, VLA-1, CD103, αvβ6, and αvβ8), aryl hydrocarbon receptor (AhR), CCR7, and the CCL27-CCR10 axis are related with the retention of TRM cells.546,547 A study using high-throughput screening on the T cell receptor (TCR) and immunostaining found that IL-17-producing resident αβT cells with psoriasis-specific antigen receptors were increased in psoriatic lesions and resolved psoriatic lesions, but not in the skin of healthy individuals. These findings indicate that TRM cells exist in the skin even when the disease is resolved, and they can lead to disease recurrence once activated.548 TRM cells can be divided into CD8+ and CD4+ subsets. The majority of CD8+ TRM cells express the cell marker CD103 (also known as integrin alpha‐E), which can bind to the E‐cadherin of keratinocytes.549 With the CD8+ TRM cells further classified as CD49+CD8+ TRM cells and CD49–CD8+ TRM cells, studies found that it was CD49–CD8+ TRM cells that drove recurrent local inflammation by producing IL-17 in the presence of certain triggers.550 Skin‐resident CD4+ T cells can also drive keratinocyte pathology through IL‐22 production.551 In summary, CD8+ TRM cells (which produce IL17) together with CD4+ TRM cells (which secrete IL-22 after the stimulation of IL-23 produced by epidermal Langerhans cells) induce keratinocyte hyperproliferation and subsequent disease relapse in resolved lesions. In addition, it is reported that through the interaction with the vascular addressin E-selectin, effector memory T (TEM) cells migrate to the skin and release IL-17, thereby participating in disease relapse.546 Lastly, it is worth mentioning that recent studies have revealed that skin epithelial stem cells (EpSCs) or basal keratinocytes with stemness can acquire long-term epigenetic memory during the course of psoriasis, which may play a potential role in the recurrence of skin inflammation in psoriasis.552 However, the above potential mechanisms still need to be verified in future works.
The current mechanistic model of recurrent psoriasis. In clinically resolved lesions, CD4+ TRMs remain in the dermis, while CD8+ TRMs and eLCs remain in the epidermis. With a disease trigger, eLCs release IL-23, leading to the release of IL-22 by CD4+ TRMs, thereby further inducing the hyperproliferation and inflammation of keratinocytes. CD49– CD8+ TRM cells also produce IL-17 to drive recurrent local inflammation. After interacting with the vascular addressin E-selectin, TEM cells migrate to the skin and release IL-17, thereby participating in disease recurrence. Created with BioRender.com
Conclusion and future prospects
Psoriasis is a common, chronic, inflammatory skin disease with a high burden on individuals, health systems, and society worldwide, suggesting the urgent need to comprehensively understand the mechanisms driving the pathogenesis of psoriasis. This review focused on altered signaling pathways involved in psoriasis pathogenesis. In the past decade, the immunopathology and pathogenesis of psoriasis have been gradually uncovered, and the development of therapeutic approaches has gained revolutionary progress. Targeted therapies with biologics have brought new hope to patients with psoriasis due to their effectiveness in modifying the disease or, potentially, even curing it by preventing the accumulation of tissue-resident memory T cells in the skin. Nevertheless, there are still many unsolved issues in psoriasis, with the adverse effects of medications and disease relapse after the discontinuation of treatment being the most prominent ones. With a deeper understanding of the mechanisms of disease recurrence, it is reasonable to believe that early treatment to prevent the emergence and spreading of issue-resident memory T cells is highly desirable. Latest research has found that IL-23 produced by CD301b+ cells can promote in situ proliferation of TRM cells, indicating that IL-23 inhibitors or new therapies targeting CD301b may be a promising strategy to cope with disease recurrence and to maintain the therapeutic effect.553 However, the clinical translation of novel therapeutic approaches still needs great efforts in the future.
Although great progress has been made in the understanding and treatment of psoriasis, further research is still needed. Future, whether genetic indicators and biomarkers can be used as predictors for psoriasis to facilitate early diagnosis and intervention is another major focus of future studies. Moreover, despite the discovery of multiple potential metabolic molecules, there is no comprehensive understanding of metabolic effects in psoriasis. With new multi-omics tools such as single-cell metabolomics and spatial metabolomics emergence, it is possible and necessary to construct a multi-dimensional metabolic network of psoriasis through algorithm innovation and multi-omics joint analysis. Targeting metabolism therapy is still limited, possibly related to metabolic heterogeneity, flexibility, and unpredictable adverse effects. Hence, identifying more desirable metabolic targets will be a crucial step forward. We believe that focusing on psoriatic metabolism and establishing a psoriasis network centered on metabolism will be the hot topic in the next decade. Another challenge in the future is to find effective therapies to manage psoriatic comorbidities, especially metabolic and cardiovascular disorders. Last but not least, attention is also needed to the relatively rare subgroups of psoriasis, such as palmoplantar psoriasis and pustular psoriasis, which are poorly responsive to current treatment options.
Change history
22 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41392-023-01733-9
References
Icen, M. et al. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J. Am. Acad. Dermatol. 60, 394–401 (2009).
Damiani, G. et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front. Med. 8, 743180 (2021).
Parisi, R. et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 369, m1590 (2020).
Meyer, N. et al. Psoriasis: an epidemiological evaluation of disease burden in 590 patients. J. Eur. Acad. Dermatol. Venereol. 24, 1075–1082 (2010).
Villacorta, R. et al. A multinational assessment of work-related productivity loss and indirect costs from a survey of patients with psoriasis. Br. J. Dermatol. 183, 548–558 (2020).
Cao, F. et al. Global burden and cross-country inequalities in autoimmune diseases from 1990 to 2019. Autoimmun. Rev. 22, 103326 (2023).
Wang, H. M., Xu, J. M. & Jin, H. Z. Characteristics and burdens of disease in patients from beijing with generalized pustular psoriasis and palmoplantar pustulosis: multicenter retrospective cohort study using a regional database. Am. J. Clin. Dermatol. https://doi.org/10.1007/s40257-023-00807-2 (2023).
Pilon, D. et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J. Med Econ. 22, 196–203 (2019).
Singh, S., Taylor, C., Kornmehl, H. & Armstrong, A. W. Psoriasis and suicidality: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 77, 425–440.e422 (2017).
Matterne, U., Baumeister, S. E. & Apfelbacher, C. J. Suicidality and risk of suicidality in psoriasis: a critical appraisal of two systematic reviews and meta-analyses. Br. J. Dermatol. 181, 717–721 (2019).
Armstrong, A. W. & Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA 323, 1945–1960 (2020).
Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E. & Barker, J. Psoriasis. Lancet 397, 1301–1315 (2021).
Patrick, M. T. et al. Shared genetic risk factors and causal association between psoriasis and coronary artery disease. Nat. Commun. 13, 6565 (2022).
Veale, D. J. & Fearon, U. The pathogenesis of psoriatic arthritis. Lancet 391, 2273–2284 (2018).
Yan, D., Afifi, L., Jeon, C., Cordoro, K. M. & Liao, W. A cross-sectional study of psoriasis triggers among different ethno-racial groups. J. Am. Acad. Dermatol. 77, 756–758.e751 (2017).
Twelves, S. et al. Clinical and genetic differences between pustular psoriasis subtypes. J. Allergy Clin. Immunol. 143, 1021–1026 (2019).
FitzGerald, O. et al. Psoriatic arthritis. Nat. Rev. Dis. Prim. 7, 59 (2021).
Chalmers, R., O’Sullivan, T., Owen, C. M. & Griffiths, C. E. WITHDRAWN: interventions for guttate psoriasis. Cochrane Database Syst. Rev. 4, Cd001213 (2019).
Carrasquillo, O. Y. et al. Treatment of erythrodermic psoriasis with biologics: a systematic review. J. Am. Acad. Dermatol. 83, 151–158 (2020).
Elmets, C. A. et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J. Am. Acad. Dermatol. 80, 1073–1113 (2019).
Kaushik, S. B. & Lebwohl, M. G. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol. 80, 27–40 (2019).
Korman, N. J. Management of psoriasis as a systemic disease: what is the evidence? Br. J. Dermatol. 182, 840–848 (2020).
Kim, W. B., Jerome, D. & Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Phys. 63, 278–285 (2017).
Boehncke, W. H. & Schön, M. P. Psoriasis. Lancet 386, 983–994 (2015).
Bronckers, I. M., Paller, A. S., van Geel, M. J., van de Kerkhof, P. C. & Seyger, M. M. Psoriasis in children and adolescents: diagnosis, management and comorbidities. Paediatr. Drugs 17, 373–384 (2015).
van de Kerkhof, P. C. From empirical to pathogenesis-based treatments for psoriasis. J. Invest. Dermatol. 142, 1778–1785 (2022).
Rendon, A. & Schäkel, K. Psoriasis pathogenesis and treatment. Int J. Mol. Sci. 20, 1475 (2019).
Raychaudhuri, S. P., Wilken, R., Sukhov, A. C., Raychaudhuri, S. K. & Maverakis, E. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J. Autoimmun. 76, 21–37 (2017).
Coates, L. C. et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 18, 465–479 (2022).
Ghoreschi, K., Balato, A., Enerbäck, C. & Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 397, 754–766 (2021).
Kumar, R., Theiss, A. L. & Venuprasad, K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 42, 1037–1050 (2021).
Conrad, C. & Gilliet, M. Psoriasis: from pathogenesis to targeted therapies. Clin. Rev. Allergy Immunol. 54, 102–113 (2018).
Deng, Y., Chang, C. & Lu, Q. The inflammatory response in psoriasis: a comprehensive review. Clin. Rev. Allergy Immunol. 50, 377–389 (2016).
Vičić, M., Kaštelan, M., Brajac, I., Sotošek, V. & Massari, L. P. Current concepts of psoriasis immunopathogenesis. Int J. Mol. Sci. 22, 11574 (2021).
Chandra, A., Ray, A., Senapati, S. & Chatterjee, R. Genetic and epigenetic basis of psoriasis pathogenesis. Mol. Immunol. 64, 313–323 (2015).
Trowbridge, R. M. & Pittelkow, M. R. Epigenetics in the pathogenesis and pathophysiology of psoriasis vulgaris. J. Drugs Dermatol 13, 111–118 (2014).
Blunder, S., Pavel, P., Minzaghi, D. & Dubrac, S. PPARdelta in affected atopic dermatitis and psoriasis: a possible role in metabolic reprograming. Int J. Mol. Sci. 22, 7354 (2021).
Lian, N., Shi, L. Q., Hao, Z. M. & Chen, M. Research progress and perspective in metabolism and metabolomics of psoriasis. Chin. Med. J. 133, 2976–2986 (2020).
Jiang, B., Zhang, H., Wu, Y. & Shen, Y. Single-cell immune ecosystem and metabolism reprogramming imprinted by psoriasis niche. Ann. Transl. Med. 10, 837 (2022).
Nair, R. P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat. Genet. 41, 199–204 (2009).
Hüffmeier, U. et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 42, 996–999 (2010).
Cargill, M. et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 80, 273–290 (2007).
Nair, R. P. et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J. Invest. Dermatol. 128, 1653–1661 (2008).
Langley, R. G. et al. Secukinumab in plaque psoriasis-results of two phase 3 trials. N. Engl. J. Med. 371, 326–338 (2014).
Blauvelt, A. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J. Am. Acad. Dermatol. 76, 405–417 (2017).
Merola, J. F. et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 401, 38–48 (2023).
Griffiths, C. E. et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N. Engl. J. Med. 362, 118–128 (2010).
Duvallet, E., Semerano, L., Assier, E., Falgarone, G. & Boissier, M. C. Interleukin-23: a key cytokine in inflammatory diseases. Ann. Med. 43, 503–511 (2011).
Teng, M. W. et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 21, 719–729 (2015).
Yawalkar, N., Tscharner, G. G., Hunger, R. E. & Hassan, A. S. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J. Dermatol. Sci. 54, 99–105 (2009).
Sun, L., Su, Y., Jiao, A., Wang, X. & Zhang, B. T cells in health and disease. Sig. Transduct. Target Ther. 8, 235 (2023).
Li, Y., Yu, X., Ma, Y. & Hua, S. IL-23 and dendritic cells: what are the roles of their mutual attachment in immune response and immunotherapy? Cytokine 120, 78–84 (2019).
Liu, T. et al. The IL-23/IL-17 pathway in inflammatory skin diseases: from bench to bedside. Front. Immunol. 11, 594735 (2020).
Gu, C., Wu, L. & Li, X. IL-17 family: cytokines, receptors and signaling. Cytokine 64, 477–485 (2013).
Johansen, C. et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 160, 319–324 (2009).
Johnston, A. et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol. 190, 2252–2262 (2013).
Martin, D. A. et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J. Invest. Dermatol. 133, 17–26 (2013).
Blauvelt, A. & Chiricozzi, A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 55, 379–390 (2018).
Chang, S. H. & Dong, C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440 (2007).
Bertelsen, T., Iversen, L. & Johansen, C. The human IL-17A/F heterodimer regulates psoriasis-associated genes through IκBζ. Exp. Dermatol. 27, 1048–1052 (2018).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in health and disease. Immunity 50, 892–906 (2019).
Keijsers, R. R., Joosten, I., van Erp, P. E., Koenen, H. J. & van de Kerkhof, P. C. Cellular sources of IL-17 in psoriasis: a paradigm shift? Exp. Dermatol. 23, 799–803 (2014).
Brembilla, N. C., Senra, L. & Boehncke, W. H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 9, 1682 (2018).
Knizkova, D. et al. CMTM4 is a subunit of the IL-17 receptor and mediates autoimmune pathology. Nat. Immunol. 23, 1644–1652 (2022).
Furue, M., Furue, K., Tsuji, G. & Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int J. Mol. Sci. 21, 1275 (2020).
Lo, Y. H. et al. Galectin-8 is upregulated in keratinocytes by IL-17A and promotes proliferation by regulating mitosis in psoriasis. J. Invest. Dermatol. 141, 503–511.e509 (2021).
Christmann, C. et al. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front. Immunol. 11, 599947 (2020).
Ekman, A. K., Bivik Eding, C., Rundquist, I. & Enerbäck, C. IL-17 and IL-22 promote keratinocyte stemness in the germinative compartment in psoriasis. J. Invest. Dermatol. 139, 1564–1573.e1568 (2019).
Xu, X. et al. Interleukin-17A drives IL-19 and IL-24 expression in skin stromal cells regulating keratinocyte proliferation. Front. Immunol. 12, 719562 (2021).
Liu, Y. et al. A novel role of IL-17A in contributing to the impaired suppressive function of Tregs in psoriasis. J. Dermatol. Sci. 101, 84–92 (2021).
Garzorz-Stark, N. & Eyerich, K. Psoriasis pathogenesis: keratinocytes are back in the spotlight. J. Invest. Dermatol. 139, 995–996 (2019).
Ramirez-Carrozzi, V. et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 12, 1159–1166 (2011).
Borowczyk, J. et al. IL-17E (IL-25) and IL-17A differentially affect the functions of human keratinocytes. J. Invest. Dermatol. 140, 1379–1389.e1372 (2020).
Reich, K. et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br. J. Dermatol. 167, 180–190 (2012).
McGeough, M. D. et al. TNF regulates transcription of NLRP3 inflammasome components and inflammatory molecules in cryopyrinopathies. J. Clin. Invest. 127, 4488–4497 (2017).
Grine, L., Dejager, L., Libert, C. & Vandenbroucke, R. E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 26, 25–33 (2015).
Hu, P. et al. The role of helper T cells in psoriasis. Front. Immunol. 12, 788940 (2021).
Kristensen, M. et al. Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin. Exp. Immunol. 94, 354–362 (1993).
Psarras, A. et al. TNF-α regulates human plasmacytoid dendritic cells by suppressing IFN-α production and enhancing T cell activation. J. Immunol. 206, 785–796 (2021).
Li, S. J., Perez-Chada, L. M. & Merola, J. F. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J. Psoriasis Psoriatic Arthritis 4, 70–80 (2019).
Zaba, L. C. et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J. Exp. Med. 204, 3183–3194 (2007).
Schottelius, A. J. et al. Biology of tumor necrosis factor-alpha- implications for psoriasis. Exp. Dermatol. 13, 193–222 (2004).
Chiricozzi, A. et al. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687 (2011).
Gupta, R. K. et al. TWEAK functions with TNF and IL-17 on keratinocytes and is a potential target for psoriasis therapy. Sci. Immunol. 6, eabi8823 (2021).
Richmond, J. M., Strassner, J. P., Essien, K. I. & Harris, J. E. T-cell positioning by chemokines in autoimmune skin diseases. Immunol. Rev. 289, 186–204 (2019).
Meehan, E. V. & Wang, K. Interleukin-17 family cytokines in metabolic disorders and cancer. Genes 13, 1643 (2022).
Kim, K. E., Houh, Y., Park, H. J. & Cho, D. Therapeutic effects of erythroid differentiation regulator 1 on imiquimod-induced psoriasis-like skin inflammation. Int J. Mol. Sci. 17, 244 (2016).
Mabuchi, T. et al. CCR6 is required for epidermal trafficking of γδ-T cells in an IL-23-induced model of psoriasiform dermatitis. J. Invest. Dermatol. 133, 164–171 (2013).
Schutyser, E., Struyf, S. & Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 (2003).
Singh, S. P., Zhang, H. H., Foley, J. F., Hedrick, M. N. & Farber, J. M. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 180, 214–221 (2008).
Shin, J. W. et al. Keratinocyte transglutaminase 2 promotes CCR6(+) γδT-cell recruitment by upregulating CCL20 in psoriatic inflammation. Cell Death Dis. 11, 301 (2020).
Harper, E. G. et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J. Invest. Dermatol. 129, 2175–2183 (2009).
Kennedy-Crispin, M. et al. Human keratinocytes’ response to injury upregulates CCL20 and other genes linking innate and adaptive immunity. J. Invest. Dermatol. 132, 105–113 (2012).
Furue, K. et al. Cyto/chemokine profile of in vitro scratched keratinocyte model: implications of significant upregulation of CCL20, CXCL8 and IL36G in Koebner phenomenon. J. Dermatol Sci. 94, 244–251 (2019).
Johnston, A. et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J. Allergy Clin. Immunol. 140, 109–120 (2017).
Neurath, M. F. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. 55, 70–79 (2020).
Blumberg, H. et al. IL-1RL2 and its ligands contribute to the cytokine network in psoriasis. J. Immunol. 185, 4354–4362 (2010).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Johnston, A. et al. IL-1F5, -F6, -F8, and -F9: a novel IL-1 family signaling system that is active in psoriasis and promotes keratinocyte antimicrobial peptide expression. J. Immunol. 186, 2613–2622 (2011).
Traks, T. et al. Polymorphisms in IL36G gene are associated with plaque psoriasis. BMC Med. Genet. 20, 10 (2019).
Marrakchi, S. et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 365, 620–628 (2011).
Onoufriadis, A. et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 89, 432–437 (2011).
Zuo, X. et al. Whole-exome SNP array identifies 15 new susceptibility loci for psoriasis. Nat. Commun. 6, 6793 (2015).
Hébert, H. L. et al. Polymorphisms in IL-1B distinguish between psoriasis of early and late onset. J. Invest. Dermatol. 134, 1459–1462 (2014).
Gresnigt, M. S. & van de Veerdonk, F. L. Biology of IL-36 cytokines and their role in disease. Semin. Immunol. 25, 458–465 (2013).
Towne, J. E. et al. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36Ra) activity. J. Biol. Chem. 286, 42594–42602 (2011).
Henry, C. M. et al. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 14, 708–722 (2016).
Clancy, D. M. et al. Extracellular neutrophil proteases are efficient regulators of IL-1, IL-33, and IL-36 cytokine activity but poor effectors of microbial killing. Cell Rep. 22, 2937–2950 (2018).
Li, N. et al. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ induction in human epidermal keratinocytes. J. Immunol. 193, 5140–5148 (2014).
Fukaura, R. & Akiyama, M. Targeting IL-36 in inflammatory skin diseases. BioDrugs 37, 279–293 (2023).
Carrier, Y. et al. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J. Invest. Dermatol. 131, 2428–2437 (2011).
Madonna, S., Girolomoni, G., Dinarello, C. A. & Albanesi, C. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J. Mol. Sci. 20, 3318 (2019).
Foster, A. M. et al. IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J. Immunol. 192, 6053–6061 (2014).
Ni, X. et al. IL-17D-induced inhibition of DDX5 expression in keratinocytes amplifies IL-36R-mediated skin inflammation. Nat. Immunol. 23, 1577–1587 (2022).
Iznardo, H. & Puig, L. The interleukin-1 family cytokines in psoriasis: pathogenetic role and therapeutic perspectives. Expert Rev. Clin. Immunol. 17, 187–199 (2021).
Cai, Y. et al. A critical role of the IL-1β-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J. Invest. Dermatol. 139, 146–156 (2019).
Negishi, H., Taniguchi, T. & Yanai, H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harb. Perspect. Biol. 10, a028423 (2018).
Mora-Arias, T. & Amezcua-Guerra, L. M. Type III interferons (Lambda Interferons) in rheumatic autoimmune diseases. Arch. Immunol. Ther. Exp. 68, 1 (2020).
Guo, H. et al. SCARB2/LIMP-2 regulates IFN production of plasmacytoid dendritic cells by mediating endosomal translocation of TLR9 and nuclear translocation of IRF7. J. Immunol. 194, 4737–4749 (2015).
Zhang, L. J. Type1 interferons potential initiating factors linking skin wounds with psoriasis pathogenesis. Front. Immunol. 10, 1440 (2019).
Toussirot, E., Béreau, M., Bossert, M., Malkoun, I. & Lohse, A. Occurrence of psoriatic arthritis during interferon beta 1a treatment for multiple sclerosis. Case Rep. Rheumatol. 2014, 949317 (2014).
Millán-Pascual, J., Turpín-Fenoll, L., Del Saz-Saucedo, P., Rueda-Medina, I. & Navarro-Muñoz, S. Psoriasis during natalizumab treatment for multiple sclerosis. J. Neurol. 259, 2758–2760 (2012).
Orzan, O. A. et al. An insight on the possible association between inflammatory bowel disease and biologic therapy with IL-17 inhibitors in psoriasis patients. Pharmaceutics 15, 2171 (2023).
Zhou, S. & Yao, Z. Roles of infection in psoriasis. Int J. Mol. Sci. 23, 6955 (2022).
Johnson-Huang, L. M. et al. A single intradermal injection of IFN-γ induces an inflammatory state in both non-lesional psoriatic and healthy skin. J. Invest. Dermatol. 132, 1177–1187 (2012).
Abdallah, M. A., Abdel-Hamid, M. F., Kotb, A. M. & Mabrouk, E. A. Serum interferon-gamma is a psoriasis severity and prognostic marker. Cutis 84, 163–168 (2009).
Albanesi, C., Madonna, S., Gisondi, P. & Girolomoni, G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front. Immunol. 9, 1549 (2018).
Zhou, X., Chen, Y., Cui, L., Shi, Y. & Guo, C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 13, 81 (2022).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Zhang, L. J. et al. Antimicrobial Peptide LL37 and MAVS Signaling Drive Interferon-β Production by Epidermal Keratinocytes during Skin Injury. Immunity 45, 119–130 (2016).
Simmons, D. P. et al. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J. Immunol. 188, 3116–3126 (2012).
Tohyama, M. et al. IFN-α enhances IL-22 receptor expression in keratinocytes: a possible role in the development of psoriasis. J. Invest. Dermatol. 132, 1933–1935 (2012).
Georgescu, S. R. et al. Advances in understanding the immunological pathways in psoriasis. Int J. Mol. Sci. 20, 739 (2019).
Young, H. A. Unraveling the pros and cons of interferon-gamma gene regulation. Immunity 24, 506–507 (2006).
Ziblat, A. et al. Interleukin (IL)-23 stimulates IFN-γ secretion by CD56(bright) natural killer cells and enhances IL-18-driven dendritic cells activation. Front. Immunol. 8, 1959 (2017).
Kryczek, I. et al. Induction of IL-17 + T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J. Immunol. 181, 4733–4741 (2008).
Sabat, R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 21, 315–324 (2010).
Lo, Y. H. et al. Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J. Dermatol. Sci. 58, 225–227 (2010).
Liu, H. et al. The expression of interleukin-22 and S100A7, A8, A9 mRNA in patients with psoriasis vulgaris. J. Huazhong Univ. Sci. Technol. Med. Sci. 27, 605–607 (2007).
Caproni, M. et al. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J. Clin. Immunol. 29, 210–214 (2009).
Meephansan, J., Ruchusatsawat, K., Sindhupak, W., Thorner, P. S. & Wongpiyabovorn, J. Effect of methotrexate on serum levels of IL-22 in patients with psoriasis. Eur. J. Dermatol. 21, 501–504 (2011).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Fujita, H. et al. Human Langerhans cells induce distinct IL-22-producing CD4 + T cells lacking IL-17 production. Proc. Natl Acad. Sci. USA 106, 21795–21800 (2009).
Hao, J. Q. Targeting interleukin-22 in psoriasis. Inflammation 37, 94–99 (2014).
Zhang, K., Chen, L., Zhu, C., Zhang, M. & Liang, C. Current knowledge of Th22 cell and IL-22 functions in infectious diseases. Pathogens 12, 176 (2023).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Wolk, K. et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J. Mol. Med. 87, 523–536 (2009).
Saggini, A., Chimenti, S. & Chiricozzi, A. IL-6 as a druggable target in psoriasis: focus on pustular variants. J. Immunol. Res. 2014, 964069 (2014).
Sobolev, V. V., Denisova, E. V., Chebysheva, S. N., Geppe, N. A. & Korsunskaya, I. M. IL-6 gene expression as a marker of pathological state in psoriasis and psoriatic arthritis. Bull. Exp. Biol. Med. 173, 77–80 (2022).
Fujishima, S. et al. Involvement of IL-17F via the induction of IL-6 in psoriasis. Arch. Dermatol. Res. 302, 499–505 (2010).
Blauvelt, A. IL-6 differs from TNF-α: unpredicted clinical effects caused by IL-6 blockade in psoriasis. J. Invest. Dermatol. 137, 541–542 (2017).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Rose-John, S., Jenkins, B. J., Garbers, C., Moll, J. M. & Scheller, J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat. Rev. Immunol. 17, 1–16 (2023).
Camporeale, A. & Poli, V. IL-6, IL-17 and STAT3: a holy trinity in auto-immunity? Front. Biosci. 17, 2306–2326 (2012).
Bodian, M. et al. Fatal evolution of a huge right atrial free-floating thrombus. Clin. Case Rep. 1, 63–65 (2013).
Fritz, Y. et al. Induction of alternative proinflammatory cytokines accounts for sustained psoriasiform skin inflammation in IL-17C + IL-6KO mice. J. Invest. Dermatol. 137, 696–705 (2017).
Midde, H. S. et al. Interleukin-9 serves as a key link between systemic inflammation and angiogenesis in psoriasis. Clin. Exp. Dermatol. 46, 50–57 (2021).
Schlapbach, C. et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci. Transl. Med. 6, 219ra218 (2014).
Theoharides, T. C. et al. IL-33 augments substance P-induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl Acad. Sci. USA 107, 4448–4453 (2010).
Shakerian, L. et al. IL-33/ST2 axis in autoimmune disease. Cytokine 158, 156015 (2022).
Zhou, X. et al. IL-33-mediated activation of mast cells is involved in the progression of imiquimod-induced psoriasis-like dermatitis. Cell Commun. Signal 21, 52 (2023).
Chen, Z. et al. Interleukin-33 alleviates psoriatic inflammation by suppressing the T helper type 17 immune response. Immunology 160, 382–392 (2020).
Suzuki, K. et al. NF-κB1 Contributes to Imiquimod-Induced Psoriasis-Like Skin Inflammation by Inducing Vγ4(+)Vδ4(+)γδT17 Cells. J. Invest. Dermatol. 142, 1639–1649.e1635 (2022).
Andres-Ejarque, R. et al. Enhanced NF-κB signaling in type-2 dendritic cells at baseline predicts non-response to adalimumab in psoriasis. Nat. Commun. 12, 4741 (2021).
Zhang, H. & Sun, S. C. NF-κB in inflammation and renal diseases. Cell Biosci. 5, 63 (2015).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Sig. Transduct. Target Ther. 2, 17023 (2017).
Yu, H., Lin, L., Zhang, Z., Zhang, H. & Hu, H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target Ther. 5, 209 (2020).
Goldminz, A. M., Au, S. C., Kim, N., Gottlieb, A. B. & Lizzul, P. F. NF-κB: an essential transcription factor in psoriasis. J. Dermatol. Sci. 69, 89–94 (2013).
Stuart, P. E. et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat. Genet. 42, 1000–1004 (2010).
Ali, F. R. & Warren, R. B. Psoriasis and susceptibility to other autoimmune diseases: an outline for the clinician. Expert Rev. Clin. Immunol. 9, 99–101 (2013).
Lorenz, V. N., Schön, M. P. & Seitz, C. S. c-Rel downregulation affects cell cycle progression of human keratinocytes. J. Invest. Dermatol 134, 415–422 (2014).
Fan, T. et al. Treating psoriasis by targeting its susceptibility gene Rel. Clin. Immunol. 165, 47–54 (2016).
Dainichi, T., Matsumoto, R., Mostafa, A. & Kabashima, K. Immune control by TRAF6-mediated pathways of epithelial cells in the EIME (epithelial immune microenvironment). Front. Immunol. 10, 1107 (2019).
Qu, F. et al. TRAF6-dependent Act1 phosphorylation by the IκB kinase-related kinases suppresses interleukin-17-induced NF-κB activation. Mol. Cell Biol. 32, 3925–3937 (2012).
Tanaka, M. et al. Essential role of CARD14 in murine experimental psoriasis. J. Immunol. 200, 71–81 (2018).
Van Nuffel, E. et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J. Invest. Dermatol. 137, 569–575 (2017).
Jordan, C. T. et al. PSORS2 is due to mutations in CARD14. Am. J. Hum. Genet. 90, 784–795 (2012).
Tsoi, L. C. et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 44, 1341–1348 (2012).
Jordan, C. T. et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am. J. Hum. Genet. 90, 796–808 (2012).
Wang, M. et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity 49, 66–79.e65 (2018).
Mellett, M. et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J. Invest. Dermatol. 138, 2010–2023 (2018).
Liang, Y., Sarkar, M. K., Tsoi, L. C. & Gudjonsson, J. E. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr. Opin. Immunol. 49, 1–8 (2017).
Mauro, C. et al. ABIN-1 binds to NEMO/IKKgamma and co-operates with A20 in inhibiting NF-kappaB. J. Biol. Chem. 281, 18482–18488 (2006).
Ramirez, V. P., Gurevich, I. & Aneskievich, B. J. Emerging roles for TNIP1 in regulating post-receptor signaling. Cytokine Growth Factor Rev. 23, 109–118 (2012).
Guo, G. et al. Gfi1 and Zc3h12c orchestrate a negative feedback loop that inhibits NF-kB activation during inflammation in macrophages. Mol. Immunol. 128, 219–226 (2020).
Liu, L. et al. Zc3h12c inhibits vascular inflammation by repressing NF-κB activation and pro-inflammatory gene expression in endothelial cells. Biochem. J. 451, 55–60 (2013).
Bousoik, E. & Montazeri Aliabadi, H. “Do We Know Jack” about JAK? A closer look at JAK/STAT signaling pathway. Front. Oncol. 8, 287 (2018).
Winthrop, K. L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13, 234–243 (2017).
Krueger, J. G., McInnes, I. B. & Blauvelt, A. Tyrosine kinase 2 and Janus kinase‒signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J. Am. Acad. Dermatol. 86, 148–157 (2022).
Damsky, W. & King, B. A. JAK inhibitors in dermatology: the promise of a new drug class. J. Am. Acad. Dermatol. 76, 736–744 (2017).
Nada, H. R., El Sharkawy, D. A., Elmasry, M. F., Rashed, L. A. & Mamdouh, S. Expression of Janus Kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch. Dermatol. Res. 310, 39–46 (2018).
Ishizaki, M. et al. Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int. Immunol. 26, 257–267 (2014).
Tomar, Y., Gorantla, S. & Singhvi, G. Insight into the pivotal role of signaling pathways in psoriasis pathogenesis, potential therapeutic molecules and drug delivery approaches. Drug Discov. Today 28, 103465 (2023).
Hald, A. et al. STAT1 expression and activation is increased in lesional psoriatic skin. Br. J. Dermatol. 168, 302–310 (2013).
Sano, S. et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 11, 43–49 (2005).
Greb, J. E. et al. Psoriasis. Nat. Rev. Dis. Prim. 2, 16082 (2016).
Kim, E. K. & Choi, E. J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 1802, 396–405 (2010).
Kyriakis, J. M. & Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 92, 689–737 (2012).
Yu, X. J. et al. Expression and localization of the activated mitogen-activated protein kinase in lesional psoriatic skin. Exp. Mol. Pathol. 83, 413–418 (2007).
Krajina, I., Stupin, A., Šola, M. & Mihalj, M. Oxidative stress induced by high salt diet-possible implications for development and clinical manifestation of cutaneous inflammation and endothelial dysfunction in Psoriasis vulgaris. Antioxidants 11, 1269 (2022).
Pasparakis, M., Haase, I. & Nestle, F. O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 14, 289–301 (2014).
Hammouda, M. B., Ford, A. E., Liu, Y. & Zhang, J. Y. The JNK signaling pathway in inflammatory skin disorders and cancer. Cells 9, 857 (2020).
Liang, J. et al. IL-22 down-regulates Cx43 expression and decreases gap junctional intercellular communication by activating the JNK pathway in psoriasis. J. Invest. Dermatol. 139, 400–411 (2019).
Chen, L., Wu, J., Ren, W., Yang, X. & Shen, Z. c-Jun N-terminal kinase (JNK)-phospho-c-JUN (ser63/73) pathway is essential for FOXP3 nuclear translocation in psoriasis. J. Dermatol. Sci. 69, 114–121 (2013).
Afonina, I. S. et al. The paracaspase MALT1 mediates CARD14-induced signaling in keratinocytes. EMBO Rep. 17, 914–927 (2016).
Mose, M., Kang, Z., Raaby, L., Iversen, L. & Johansen, C. TNFα- and IL-17A-mediated S100A8 expression is regulated by p38 MAPK. Exp. Dermatol. 22, 476–481 (2013).
Hau, C. S. et al. Visfatin enhances the production of cathelicidin antimicrobial peptide, human β-defensin-2, human β-defensin-3, and S100A7 in human keratinocytes and their orthologs in murine imiquimod-induced psoriatic skin. Am. J. Pathol. 182, 1705–1717 (2013).
Sun, Y. et al. CCN1 promotes IL-1β production in keratinocytes by activating p38 MAPK signaling in psoriasis. Sci. Rep. 7, 43310 (2017).
Funding, A. T., Johansen, C., Kragballe, K. & Iversen, L. Mitogen- and stress-activated protein kinase 2 and cyclic AMP response element binding protein are activated in lesional psoriatic epidermis. J. Invest. Dermatol. 127, 2012–2019 (2007).
Wu, Y. et al. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Commun. Signal 16, 58 (2018).
Chen, J. G., Fan, H. Y., Wang, T., Lin, L. Y. & Cai, T. G. Silencing KRT16 inhibits keratinocyte proliferation and VEGF secretion in psoriasis via inhibition of ERK signaling pathway. Kaohsiung J. Med. Sci. 35, 284–296 (2019).
Yang, J., Sun, L., Han, J., Zheng, W. & Peng, W. DUSP1/MKP-1 regulates proliferation and apoptosis in keratinocytes through the ERK/Elk-1/Egr-1 signaling pathway. Life Sci. 223, 47–53 (2019).
Mercurio, L., Albanesi, C. & Madonna, S. Recent updates on the involvement of PI3K/AKT/mTOR molecular cascade in the pathogenesis of hyperproliferative skin disorders. Front. Med. 8, 665647 (2021).
Chamcheu, J. C. et al. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in human psoriasis and imiquimod-induced murine psoriasiform dermatitis model. Acta Derm. Venereol. 96, 854–856 (2016).
Huang, T., Lin, X., Meng, X. & Lin, M. Phosphoinositide-3 kinase/protein kinase-B/mammalian target of rapamycin pathway in psoriasis pathogenesis. A potential therapeutic target? Acta Derm. Venereol. 94, 371–379 (2014).
Zhang, Q. et al. Signaling pathways and targeted therapy for myocardial infarction. Sig. Transduct. Target Ther. 7, 78 (2022).
Zhang, M. & Zhang, X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 311, 83–91 (2019).
Teng, Y. et al. The PI3K/Akt pathway: emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 10, 1219 (2021).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Kodani, N. & Nakae, J. Tissue-specific metabolic regulation of FOXO-binding protein: FOXO does not act alone. Cells 9, 702 (2020).
Wang, H., Ran, L. W., Hui, K., Wang, X. Y. & Zheng, Y. Expressions of survivin, PI3K and AKT in keratinocytes in skin lesions and their pathogenic role in psoriasis vulgaris. Nan Fang. Yi Ke Da Xue Xue Bao 37, 1512–1516 (2017).
Zhang, X. Y. et al. Increased activities of Akt in psoriatic epidermis. Chin. J. Dermatol. 42, 413–416 (2009).
Ainali, C. et al. Transcriptome classification reveals molecular subtypes in psoriasis. BMC Genomics 13, 472 (2012).
Liu, Y., Luo, W. & Chen, S. Comparison of gene expression profiles reveals aberrant expression of FOXO1, Aurora A/B and EZH2 in lesional psoriatic skins. Mol. Biol. Rep. 38, 4219–4224 (2011).
Mitra, A., Raychaudhuri, S. K. & Raychaudhuri, S. P. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine 60, 38–42 (2012).
Patel, A. B., Tsilioni, I., Weng, Z. & Theoharides, T. C. TNF stimulates IL-6, CXCL8 and VEGF secretion from human keratinocytes via activation of mTOR, inhibited by tetramethoxyluteolin. Exp. Dermatol. 27, 135–143 (2018).
Chen, L., Wu, J., Pier, E., Zhao, Y. & Shen, Z. mTORC2-PKBα/Akt1 Serine 473 phosphorylation axis is essential for regulation of FOXP3 Stability by chemokine CCL3 in psoriasis. J. Invest. Dermatol. 133, 418–428 (2013).
Akinduro, O. et al. Constitutive autophagy and nucleophagy during epidermal differentiation. J. Invest. Dermatol. 136, 1460–1470 (2016).
Glaviano, A. et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22, 138 (2023).
Li, Y. et al. Downregulation of PTEN expression in psoriatic lesions. Int J. Dermatol. 53, 855–860 (2014).
Wang, R., Wang, F. F., Cao, H. W. & Yang, J. Y. MiR-223 regulates proliferation and apoptosis of IL-22-stimulated HaCat human keratinocyte cell lines via the PTEN/Akt pathway. Life Sci. 230, 28–34 (2019).
Xu, L. et al. MiR-155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed. Pharmacother. 90, 524–530 (2017).
Lu, J. et al. CircRAB3B suppresses proliferation, motility, cell cycle progression and promotes the apoptosis of IL-22-induced keratinocytes depending on the regulation of miR-1228-3p/PTEN axis in psoriasis. Autoimmunity 54, 303–312 (2021).
Gudjonsson, J. E. et al. Evidence for altered Wnt signaling in psoriatic skin. J. Invest. Dermatol. 130, 1849–1859 (2010).
Zhang, Y. et al. Wnt/β-Catenin and Wnt5a/Ca pathways regulate proliferation and apoptosis of keratinocytes in psoriasis lesions. Cell Physiol. Biochem. 36, 1890–1902 (2015).
Verma, D. et al. Differential DNA methylation of miRNA-encoding genes in psoriatic epidermis highlights the Wnt pathway. J. Invest. Dermatol. 143, 1594–1597.e14 (2023).
Müller, A. et al. The CDK4/6-EZH2 pathway is a potential therapeutic target for psoriasis. J. Clin. Invest. 130, 5765–5781 (2020).
Tsoi, L. C. et al. Enhanced meta-analysis and replication studies identify five new psoriasis susceptibility loci. Nat. Commun. 6, 7001 (2015).
Ben Abdallah, H., Johansen, C. & Iversen, L. Key signaling pathways in psoriasis: recent insights from antipsoriatic therapeutics. Psoriasis 11, 83–97 (2021).
Eyerich, K., Eyerich, S., Hiller, J., Behrendt, H. & Traidl-Hoffmann, C. Chronic mucocutaneous candidiasis, from bench to bedside. Eur. J. Dermatol. 20, 260–265 (2010).
Loo, W. J. et al. Clinical implications of targeting the JAK-STAT pathway in psoriatic disease: emphasis on the TYK2 pathway. J. Cutan. Med. Surg. 27, 3s–24s (2023).
Tang, L. et al. Transcription factor retinoid-related orphan receptor γt: a promising target for the treatment of psoriasis. Front. Immunol. 9, 1210 (2018).
Ghoreschi, K. et al. TYK2 inhibition and its potential in the treatment of chronic inflammatory immune diseases. J. Dtsch Dermatol. Ges. 19, 1409–1420 (2021).
Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Sig. Transduct. Target Ther. 6, 402 (2021).
Hu, X. & Ivashkiv, L. B. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity 31, 539–550 (2009).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016).
Brenner, D., Blaser, H. & Mak, T. W. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 15, 362–374 (2015).
Dietrich, D. & Gabay, C. Inflammation: IL-36 has proinflammatory effects in skin but not in joints. Nat. Rev. Rheumatol. 10, 639–640 (2014).
Pohla, L. et al. Hyperproliferation is the main driver of metabolomic changes in psoriasis lesional skin. Sci. Rep. 10, 3081 (2020).
Hiebert, P. & Werner, S. Targeting metabolism to treat psoriasis. Nat. Med. 24, 537–539 (2018).
Holt, V., Morén, B., Fryklund, C., Colbert, R. A. & Stenkula, K. G. Acute cytokine treatment stimulates glucose uptake and glycolysis in human keratinocytes. Cytokine 161, 156057 (2023).
Sylow, L., Kleinert, M., Richter, E. A. & Jensen, T. E. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 13, 133–148 (2017).
Hodeib, A. A., Neinaa, Y. M. E., Zakaria, S. S. & Alshenawy, H. A. Glucose transporter-1 (GLUT-1) expression in psoriasis: correlation with disease severity. Int J. Dermatol. 57, 943–951 (2018).
Abdou, A. G., Maraee, A. H., Eltahmoudy, M. & El-Aziz, R. A. Immunohistochemical expression of GLUT-1 and Ki-67 in chronic plaque psoriasis. Am. J. Dermatopathol. 35, 731–737 (2013).
Zhang, Z. et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 24, 617–627 (2018).
Tang, W. et al. HIF-1α may promote glycolysis in psoriasis vulgaris via upregulation of CD147 and GLUT1. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 46, 333–344 (2021).
Liu, Y. Z. et al. Pyruvate kinase M2 mediates glycolysis contributes to psoriasis by promoting keratinocyte proliferation. Front. Pharm. 12, 765790 (2021).
Veras, F. P. et al. Pyruvate kinase M2 mediates IL-17 signaling in keratinocytes driving psoriatic skin inflammation. Cell Rep. 41, 111897 (2022).
Kono, M. et al. Pyruvate kinase M2 is requisite for Th1 and Th17 differentiation. JCI Insight 4, e127395 (2019).
Damasceno, L. E. A. et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 217, e20190613 (2020).
Certo, M., Tsai, C. H., Pucino, V., Ho, P. C. & Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Claps, G. et al. The multiple roles of LDH in cancer. Nat. Rev. Clin. Oncol. 19, 749–762 (2022).
Le Floch, R. et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl Acad. Sci. USA 108, 16663–16668 (2011).
Baba, M., Inoue, M., Itoh, K. & Nishizawa, Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem. Biophys. Res Commun. 374, 111–116 (2008).
Walters, D. K., Arendt, B. K. & Jelinek, D. F. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle 12, 3175–3183 (2013).
Koguchi-Yoshioka, H. et al. Serum lactate dehydrogenase level as a possible predictor of treatment preference in psoriasis. J. Dermatol. Sci. 103, 109–115 (2021).
Lu, H. et al. CD147 is highly expressed on peripheral blood neutrophils from patients with psoriasis and induces neutrophil chemotaxis. J. Dermatol. 37, 1053–1056 (2010).
Peng, C. et al. Epidermal CD147 expression plays a key role in IL-22-induced psoriatic dermatitis. Sci. Rep. 7, 44172 (2017).
Okubo, A. et al. CD147 is essential for the development of psoriasis via the induction of Th17 cell differentiation. Int. J. Mol. Sci. 23, 177 (2021).
Rambold, A. S. & Pearce, E. L. Mitochondrial dynamics at the interface of immune cell metabolism and function. Trends Immunol. 39, 6–18 (2018).
Fan, J. X. et al. New conditions of HPLC analysis for separation and quantification of simple organic acids of tricarboxylic acid cycle in psoriasis. Acta Chromatographica. 33, 322–332 (2021).
Fan, J. X., et al. Quantitative detection and metabolic profile analysis of metabolites of tricarboxylic acid cycle and amino acids in psoriasis serum before and after receiving monoclonal antibody treatment by one-pot GC-MS derivatization. Int. J. Mass. Spectrom, 460 116478 (2021).
Ghoreschi, K. et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 208, 2291–2303 (2011).
Mrowietz, U. et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J. Eur. Acad. Dermatol. Venereol. 32, 3–14 (2018).
Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature 556, 501–504 (2018).
Nastasi, C. et al. Inhibition of succinate dehydrogenase activity impairs human T cell activation and function. Sci. Rep. 11, 1458 (2021).
Kamleh, M. A. et al. LC-MS metabolomics of psoriasis patients reveals disease severity-dependent increases in circulating amino acids that are ameliorated by anti-TNFα treatment. J. Proteome Res. 14, 557–566 (2015).
Chen, C. et al. Metabolomic profiling reveals amino acid and carnitine alterations as metabolic signatures in psoriasis. Theranostics 11, 754–767 (2021).
Goetzman, E. S. & Prochownik, E. V. The role for Myc in coordinating glycolysis, oxidative phosphorylation, glutaminolysis, and fatty acid metabolism in normal and neoplastic tissues. Front. Endocrinol. 9, 129 (2018).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014).
Xia, X. et al. GLS1-mediated glutaminolysis unbridled by MALT1 protease promotes psoriasis pathogenesis. J. Clin. Invest. 130, 5180–5196 (2020).
Cibrian, D. et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 17, 985–996 (2016).
Cibrian, D. et al. Targeting L-type amino acid transporter 1 in innate and adaptive T cells efficiently controls skin inflammation. J. Allergy Clin. Immunol. 145, 199–214.e111 (2020).
Poitelon, Y., Kopec, A. M. & Belin, S. Myelin fat facts: an overview of lipids and fatty acid metabolism. Cells 9, 812 (2020).
Feingold, K. R. The outer frontier: the importance of lipid metabolism in the skin. J. Lipid Res. 50, S417–S422 (2009).
Chibowska, M. [Role of serum lipids in pseriasis]. Przegl Dermatol 57, 255–260 (1970).
Hammarström, S. et al. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc. Natl Acad. Sci. USA 72, 5130–5134 (1975).
Sorokin, A. V. et al. Bioactive Lipid Mediator Profiles in Human Psoriasis Skin and Blood. J. Invest. Dermatol. 138, 1518–1528 (2018).
Kim, S. et al. Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin. Mol. Med. 12, 17–24 (2006).
Myśliwiec, H. et al. Increase in circulating sphingosine-1-phosphate and decrease in ceramide levels in psoriatic patients. Arch. Dermatol. Res. 309, 79–86 (2017).
Schaper, K. et al. Sphingosine-1-phosphate exhibits anti-proliferative and anti-inflammatory effects in mouse models of psoriasis. J. Dermatol. Sci. 71, 29–36 (2013).
Jeon, S. et al. Inhibition of sphingosine 1-phosphate lyase activates human keratinocyte differentiation and attenuates psoriasis in mice. J. Lipid Res. 61, 20–32 (2020).
Shin, S. H. et al. Inhibiting sphingosine kinase 2 derived-sphingosine-1-phosphate ameliorates psoriasis-like skin disease via blocking Th17 differentiation of naïve CD4 T lymphocytes in mice. Acta Derm. Venereol. 99, 594–601 (2019).
Vaclavkova, A. et al. Oral ponesimod in patients with chronic plaque psoriasis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 384, 2036–2045 (2014).
Liu, P. et al. Lipidomic profiling reveals metabolic signatures in psoriatic skin lesions. Clin. Immunol. 246, 109212 (2023).
Zeng, C. et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. Gigascience 6, 1–11 (2017).
Lei, L. et al. Lysophosphatidic acid mediates the pathogenesis of psoriasis by activating keratinocytes through LPAR5. Sig. Transduct. Target Ther. 6, 19 (2021).
Decara, J. et al. Peroxisome proliferator-activated receptors: experimental targeting for the treatment of inflammatory bowel diseases. Front. Pharm. 11, 730 (2020).
Schuster, C. et al. S1PR4-dependent CCL2 production promotes macrophage recruitment in a murine psoriasis model. Eur. J. Immunol. 50, 839–845 (2020).
Schaper, K., Kietzmann, M. & Bäumer, W. Sphingosine-1-phosphate differently regulates the cytokine production of IL-12, IL-23 and IL-27 in activated murine bone marrow derived dendritic cells. Mol. Immunol. 59, 10–18 (2014).
Dillmann, C. et al. S1PR4 signaling attenuates ILT 7 internalization to limit IFN-α production by human plasmacytoid dendritic cells. J. Immunol. 196, 1579–1590 (2016).
Baeyens, A. A. L. & Schwab, S. R. Finding a way out: S1P signaling and immune cell migration. Annu. Rev. Immunol. 38, 759–784 (2020).
Pietrzak, A., Michalak-Stoma, A., Chodorowska, G. & Szepietowski, J. C. Lipid disturbances in psoriasis: an update. Mediators Inflamm. 2010, 535612 (2010).
Xiang, Z., Yang, Y., Chang, C. & Lu, Q. The epigenetic mechanism for discordance of autoimmunity in monozygotic twins. J. Autoimmun. 83, 43–50 (2017).
Generali, E., Ceribelli, A., Stazi, M. A. & Selmi, C. Lessons learned from twins in autoimmune and chronic inflammatory diseases. J. Autoimmun. 83, 51–61 (2017).
Li, J. et al. Multi-omics study in monozygotic twins confirm the contribution of de novo mutation to psoriasis. J. Autoimmun. 106, 102349 (2020).
Yu, J. et al. Pathogenesis, multi-omics research, and clinical treatment of psoriasis. J. Autoimmun. 133, 102916 (2022).
Yi, J. Z. & McGee, J. S. Epigenetic-modifying therapies: an emerging avenue for the treatment of inflammatory skin diseases. Exp. Dermatol. 30, 1167–1176 (2021).
Surace, A. E. A. & Hedrich, C. M. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 10, 1525 (2019).
Mattei, A. L., Bailly, N. & Meissner, A. DNA methylation: a historical perspective. Trends Genet 38, 676–707 (2022).
Roberson, E. D. et al. A subset of methylated CpG sites differentiate psoriatic from normal skin. J. Invest. Dermatol. 132, 583–592 (2012).
Zhou, F. et al. Epigenome-wide association data implicates DNA methylation-mediated genetic risk in psoriasis. Clin. Epigenetics 8, 131 (2016).
Zhang, P. et al. Whole-genome DNA methylation in skin lesions from patients with psoriasis vulgaris. J. Autoimmun. 41, 17–24 (2013).
Zhou, F. et al. Epigenome-wide association analysis identified nine skin DNA methylation loci for psoriasis. J. Invest. Dermatol. 136, 779–787 (2016).
Zhang, P., Su, Y., Chen, H., Zhao, M. & Lu, Q. Abnormal DNA methylation in skin lesions and PBMCs of patients with psoriasis vulgaris. J. Dermatol. Sci. 60, 40–42 (2010).
Yooyongsatit, S. et al. Patterns and functional roles of LINE-1 and Alu methylation in the keratinocyte from patients with psoriasis vulgaris. J. Hum. Genet. 60, 349–355 (2015).
Suarez, N. A., Macia, A. & Muotri, A. R. LINE-1 retrotransposons in healthy and diseased human brain. Dev. Neurobiol. 78, 434–455 (2018).
Guengerich, F. P. & Cheng, Q. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharm. Rev. 63, 684–699 (2011).
Sheng, Y. et al. CYP2S1 might regulate proliferation and immune response of keratinocyte in psoriasis. Epigenetics 16, 618–628 (2021).
Wang, Z. et al. Autophagy-based unconventional secretion of HMGB1 by keratinocytes plays a pivotal role in psoriatic skin inflammation. Autophagy 17, 529–552 (2021).
Verma, D., Ekman, A. K., Bivik Eding, C. & Enerbäck, C. Genome-wide DNA methylation profiling identifies differential methylation in uninvolved psoriatic epidermis. J. Invest. Dermatol. 138, 1088–1093 (2018).
Chandra, A., Senapati, S., Roy, S., Chatterjee, G. & Chatterjee, R. Epigenome-wide DNA methylation regulates cardinal pathological features of psoriasis. Clin. Epigenetics 10, 108 (2018).
Xu, X. et al. Genome-wide DNA methylation of Munro’s microabscess reveals the epigenetic regulation in the pathogenesis of psoriasis. Front. Immunol. 13, 1057839 (2022).
Witte, K. et al. Increased presence and differential molecular imprinting of transit amplifying cells in psoriasis. J. Mol. Med. 98, 111–122 (2020).
Liu, Y., Cui, S., Sun, J., Yan, X. & Han, D. Identification of potential biomarkers for psoriasis by DNA methylation and gene expression datasets. Front. Genet. 12, 722803 (2021).
Deng, M. et al. DNA methylation markers in peripheral blood for psoriatic arthritis. J. Dermatol. Sci. 108, 39–47 (2022).
Liu, L. et al. Aberrant promoter methylation of Wnt inhibitory factor-1 gene is a potential target for treating psoriasis. Clin. Immunol. 250, 109294 (2023).
Sabari, B. R., Zhang, D., Allis, C. D. & Zhao, Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 18, 90–101 (2017).
Fu, Y., Yu, J., Li, F. & Ge, S. Oncometabolites drive tumorigenesis by enhancing protein acylation: from chromosomal remodelling to nonhistone modification. J. Exp. Clin. Cancer Res. 41, 144 (2022).
Li, X. et al. Lactate metabolism in human health and disease. Sig. Transduct. Target Ther. 7, 305 (2022).
Zheng, Q., Osunsade, A. & David, Y. Protein arginine deiminase 4 antagonizes methylglyoxal-induced histone glycation. Nat. Commun. 11, 3241 (2020).
Zhang, X. et al. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 5, 35 (2019).
Millán-Zambrano, G., Burton, A., Bannister, A. J. & Schneider, R. Histone post-translational modifications - cause and consequence of genome function. Nat. Rev. Genet. 23, 563–580 (2022).
Gibson, F. et al. Epigenetic dysregulation in autoimmune and inflammatory skin diseases. Clin. Rev. Allergy Immunol. 63, 447–471 (2022).
Zhang, P., Su, Y., Zhao, M., Huang, W. & Lu, Q. Abnormal histone modifications in PBMCs from patients with psoriasis vulgaris. Eur. J. Dermatol. 21, 552–557 (2011).
Ekman, A. K. & Enerbäck, C. Lack of preclinical support for the efficacy of histone deacetylase inhibitors in the treatment of psoriasis. Br. J. Dermatol. 174, 424–426 (2016).
Li, H. et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat. Commun. 9, 1420 (2018).
Samuelov, L. et al. Vorinostat, a histone deacetylase inhibitor, as a potential novel treatment for psoriasis. Exp. Dermatol. 31, 567–576 (2022).
Thatikonda, S., Pooladanda, V., Sigalapalli, D. K. & Godugu, C. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis. 11, 21 (2020).
Ariel, F. et al. R-loop mediated trans action of the APOLO long noncoding RNA. Mol. Cell 77, 1055–1065.e1054 (2020).
Anastasiadou, E., Jacob, L. S. & Slack, F. J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 18, 5–18 (2018).
Kopp, F. & Mendell, J. T. Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407 (2018).
Yang, L., Wilusz, J. E. & Chen, L. L. Biogenesis and regulatory roles of circular RNAs. Annu. Rev. Cell Dev. Biol. 38, 263–289 (2022).
Winkle, M., El-Daly, S. M., Fabbri, M. & Calin, G. A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov. 20, 629–651 (2021).
Wu, R. et al. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J. Clin. Invest. 128, 2551–2568 (2018).
Yang, L. et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N(6)-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 149, 2021–2033 (2022).
Agbu, P. & Carthew, R. W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 22, 425–438 (2021).
Jung, G., Hernández-Illán, E., Moreira, L., Balaguer, F. & Goel, A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 17, 111–130 (2020).
Gao, H. et al. MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab. 34, 978–990.e974 (2022).
Zhu, J. et al. Stem cell-homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci. Adv. 8, eabk0011 (2022).
Dopytalska, K., Ciechanowicz, P., Wiszniewski, K., Szymańska, E. & Walecka, I. The role of epigenetic factors in psoriasis. Int J. Mol. Sci. 22, 9294 (2021).
Li, H., Zhou, L. & Dai, J. Retinoic acid receptor-related orphan receptor RORα regulates differentiation and survival of keratinocytes during hypoxia. J. Cell Physiol. 233, 641–650 (2018).
Li, J. et al. Inhibition of miR-155 attenuates CD14(+) monocyte-mediated inflammatory response and oxidative stress in psoriasis through TLR4/MyD88/NF-κB signaling pathway. Clin. Cosmet. Investig. Dermatol 15, 193–201 (2022).
Luo, Q. et al. Silencing of miR‑155 suppresses inflammatory responses in psoriasis through inflammasome NLRP3 regulation. Int J. Mol. Med. 42, 1086–1095 (2018).
Guo, W. et al. Ebosin ameliorates psoriasis-like inflammation of mice via miR-155 targeting tnfaip3 on IL-17 pathway. Front. Immunol. 12, 662362 (2021).
Wang, H. et al. The miR-155/GATA3/IL37 axis modulates the production of proinflammatory cytokines upon TNF-α stimulation to affect psoriasis development. Exp. Dermatol. 29, 647–658 (2020).
Liu, Y. et al. MiR-155 inhibits TP53INP1 expression leading to enhanced glycolysis of psoriatic mesenchymal stem cells. J. Dermatol. Sci. 105, 142–151 (2022).
Abdallah, F. & Pichon, C. Evidence on the direct correlation between miR-31 and IL-22 axis in IMQ-induced psoriasis. Exp. Dermatol. 28, 1336–1340 (2019).
Wang, M. J. et al. Metabolic rewiring in keratinocytes by miR-31-5p identifies therapeutic intervention for psoriasis. EMBO Mol. Med. 15, e15674 (2023).
Wang, Q. et al. Levels of miR-31 and its target genes in dermal mesenchymal cells of patients with psoriasis. Int J. Dermatol. 58, 198–204 (2019).
Wang, J. K., Wang, Z. & Li, G. MicroRNA-125 in immunity and cancer. Cancer Lett. 454, 134–145 (2019).
Raaby, L. et al. Changes in mRNA expression precede changes in microRNA expression in lesional psoriatic skin during treatment with adalimumab. Br. J. Dermatol. 173, 436–447 (2015).
Su, F., Jin, L. & Liu, W. MicroRNA-125a correlates with decreased psoriasis severity and inflammation and represses keratinocyte proliferation. Dermatology 237, 568–578 (2021).
Yang, Z. et al. STAT3/SH3PXD2A-AS1/miR-125b/STAT3 positive feedback loop affects psoriasis pathogenesis via regulating human keratinocyte proliferation. Cytokine 144, 155535 (2021).
Pan, M., Huang, Y., Zhu, X., Lin, X. & Luo, D. miR‑125b‑mediated regulation of cell proliferation through the Jagged‑1/Notch signaling pathway by inhibiting BRD4 expression in psoriasis. Mol. Med. Rep. 19, 5227–5236 (2019).
Chen, H. et al. CircVAPA contributes to hyper-proliferation and inflammation of keratinocytes through miR-125b-5p/sirt6 axis in psoriasis. Int. Immunopharmacol. 115, 109632 (2023).
Chowdhari, S., Sardana, K. & Saini, N. miR-4516, a microRNA downregulated in psoriasis inhibits keratinocyte motility by targeting fibronectin/integrin α9 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 3142–3152 (2017).
Chowdhari, S. & Saini, N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J. Cell Physiol. 229, 1630–1638 (2014).
Srivastava, A. et al. Cross-talk between IFN-γ and TWEAK through miR-149 amplifies skin inflammation in psoriasis. J. Allergy Clin. Immunol. 147, 2225–2235 (2021).
Xu, J. et al. IRF3-binding lncRNA-ISIR strengthens interferon production in viral infection and autoinflammation. Cell Rep. 37, 109926 (2021).
Zou, Y. et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J. Clin. Invest. 128, 4510–4524 (2018).
Akıncılar, S. C. et al. NAIL: an evolutionarily conserved lncRNA essential for licensing coordinated activation of p38 and NFκB in colitis. Gut 70, 1857–1871 (2021).
Yan, J. et al. Long noncoding RNA expression profile and functional analysis in psoriasis. Mol. Med. Rep. 19, 3421–3430 (2019).
Tsoi, L. C. et al. Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol. 16, 24 (2015).
Li, S. et al. LncRNA NORAD engages in psoriasis by binding to miR-26a to regulate keratinocyte proliferation. Autoimmunity 54, 129–137 (2021).
Qiao, M., Li, R., Zhao, X., Yan, J. & Sun, Q. Up-regulated lncRNA-MSX2P1 promotes the growth of IL-22-stimulated keratinocytes by inhibiting miR-6731-5p and activating S100A7. Exp. Cell Res. 363, 243–254 (2018).
Duan, Q. et al. LncRNA RP6-65G23.1 accelerates proliferation and inhibits apoptosis via p-ERK1/2/p-AKT signaling pathway on keratinocytes. J. Cell Biochem. 121, 4580–4589 (2020).
Gao, J. et al. Knockdown of lncRNA MIR31HG inhibits cell proliferation in human HaCaT keratinocytes. Biol. Res. 51, 30 (2018).
Jia, H. Y. et al. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Mol. Cell Biol. 20, 46 (2019).
Lin, J. et al. LOC285194 inhibits proliferation of human keratinocytes through regulating miR-616/GATA3 pathway. Mol. Cell Probes 53, 101598 (2020).
Gupta, R. et al. Landscape of long noncoding RNAs in psoriatic and healthy skin. J. Invest. Dermatol. 136, 603–609 (2016).
He, R. et al. Identification of a long noncoding RNA TRAF3IP2-AS1 as key regulator of IL-17 signaling through the SRSF10-IRF1-Act1 axis in autoimmune diseases. J. Immunol. 206, 2353–2365 (2021).
Kristensen, L. S. et al. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691 (2019).
Liu, C. X. & Chen, L. L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Chen, L. L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490 (2020).
Li, B. et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat. Commun. 12, 295 (2021).
Liu, B. et al. An inducible circular RNA circKcnt2 inhibits ILC3 activation to facilitate colitis resolution. Nat. Commun. 11, 4076 (2020).
Qiao, M. et al. Circular RNA expression profile and analysis of their potential function in psoriasis. Cell Physiol. Biochem. 50, 15–27 (2018).
Yang, L. et al. hsa_circ_0003738 inhibits the suppressive function of tregs by targeting miR-562/IL-17A and miR-490-5p/IFN-γ signaling pathway. Mol. Ther. Nucleic Acids 21, 1111–1119 (2020).
Zaccara, S., Ries, R. J. & Jaffrey, S. R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 20, 608–624 (2019).
Oerum, S., Meynier, V., Catala, M. & Tisné, C. A comprehensive review of m6A/m6Am RNA methyltransferase structures. Nucleic Acids Res. 49, 7239–7255 (2021).
Wang, Y. N. & Jin, H. Z. Transcriptome-wide m(6)A methylation in skin lesions from patients with psoriasis vulgaris. Front. Cell Dev. Biol. 8, 591629 (2020).
Xian, J. et al. N(6)-methyladenosine-modified long non-coding RNA AGAP2-AS1 promotes psoriasis pathogenesis via miR-424-5p/AKT3 axis. J. Dermatol. Sci. 105, 27–36 (2022).
Xiao, Z. et al. METTL3-mediated m6A methylation orchestrates mRNA stability and dsRNA contents to equilibrate γδ T1 and γδ T17 cells. Cell Rep. 42, 112684 (2023).
Strauss, H. Zur Lehre von der neurogenen und der thyreogenen Glykosurie (Schluss aus No. 18.). Dtsch. Medizinische WOCHENSCHR. - DEUT MED WOCHENSCHR 23, 309–312 (1897).
Greenberg, R. et al. Comorbidities in patients with palmoplantar plaque psoriasis. J. Am. Acad. Dermatol. 84, 639–643 (2021).
Ruiz Genao, D. P. et al. Differences in epidemiology, comorbidities and treatment choice between plaque psoriasis and pustular psoriasis: results from the BIOBADADERM registry. Br. J. Dermatol. 187, 817–820 (2022).
Zhang, L., Wang, Y., Qiu, L. & Wu, J. Psoriasis and cardiovascular disease risk in European and East Asian populations: evidence from meta-analysis and Mendelian randomization analysis. BMC Med. 20, 421 (2022).
Srivastava, A. K. et al. Insights into interplay of immunopathophysiological events and molecular mechanistic cascades in psoriasis and its associated comorbidities. J. Autoimmun. 118, 102614 (2021).
Fu, Y., Lee, C. H. & Chi, C. C. Association of psoriasis with colorectal cancer. J. Am. Acad. Dermatol. 85, 1429–1436 (2021).
Vaengebjerg, S., Skov, L., Egeberg, A. & Loft, N. D. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 156, 421–429 (2020).
Quaranta, M. et al. Differential contribution of CDKAL1 variants to psoriasis, Crohn’s disease and type II diabetes. Genes Immun. 10, 654–658 (2009).
Wang, H. et al. Identification of PTPN22, ST6GAL1 and JAZF1 as psoriasis risk genes demonstrates shared pathogenesis between psoriasis and diabetes. Exp. Dermatol. 26, 1112–1117 (2017).
Buerger, C. et al. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. J. Invest Dermatol. 132, 2206–2214 (2012).
Rohm, T. V., Meier, D. T., Olefsky, J. M. & Donath, M. Y. Inflammation in obesity, diabetes, and related disorders. Immunity 55, 31–55 (2022).
Wolf, N. et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J. Med. Genet. 45, 114–116 (2008).
Cottone, M., Sapienza, C., Macaluso, F. S. & Cannizzaro, M. Psoriasis and inflammatory bowel disease. Dig. Dis. 37, 451–457 (2019).
Kakurai, M. et al. Vasoactive intestinal peptide and inflammatory cytokines enhance vascular endothelial growth factor production from epidermal keratinocytes. Br. J. Dermatol. 161, 1232–1238 (2009).
Kowalczyk, A., Kleniewska, P., Kolodziejczyk, M., Skibska, B. & Goraca, A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch. Immunol. Ther. Exp. 63, 41–52 (2015).
Alouffi, S., Faisal, M., Alatar, A. A. & Ahmad, S. Oxidative modification of LDL by various physicochemical techniques: its probable role in diabetes coupled with CVDs. Biomed. Res. Int. 2018, 7390612 (2018).
Shih, C. M. et al. The roles of lipoprotein in psoriasis. Int J. Mol. Sci. 21, 859 (2020).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Karbach, S. et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 34, 2658–2668 (2014).
Barros, G., Duran, P., Vera, I. & Bermúdez, V. Exploring the links between obesity and psoriasis: a comprehensive review. Int. J. Mol. Sci. 23, 7499 (2022).
Kong, Y. et al. New insights into different adipokines in linking the pathophysiology of obesity and psoriasis. Lipids Health Dis. 18, 171 (2019).
González-Parra, S. & Daudén, E. Psoriasis and depression: the role of inflammation. Actas Dermosifiliogr. 110, 12–19 (2019).
Lada, G., Chinoy, H., Talbot, P. S., Warren, R. B. & Kleyn, C. E. The relationship of depression and systemic inflammation in psoriasis: findings from the UK biobank. J. Invest. Dermatol. 143, 1091–1094 (2023).
Marek-Jozefowicz, L. et al. The brain-skin axis in psoriasis-psychological, psychiatric, hormonal, and dermatological aspects. Int. J. Mol. Sci. 23, 669 (2022).
Kamiya, K., Kishimoto, M., Sugai, J., Komine, M. & Ohtsuki, M. Risk factors for the development of psoriasis. Int. J. Mol. Sci. 20, 4347 (2019).
Pace, T. W. & Miller, A. H. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann. NY Acad. Sci. 1179, 86–105 (2009).
Svoboda, S. A., Ghamrawi, R. I., Owusu, D. A. & Feldman, S. R. Treatment goals in psoriasis: which outcomes matter most? Am. J. Clin. Dermatol. 21, 505–511 (2020).
Nast, A. & Schmitt, J. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 68, 1040–1041 (2013).
Nast, A. et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J. Eur. Acad. Dermatol. Venereol. 34, 2461–2498 (2020).
McInnes, I. B. et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 395, 1496–1505 (2020).
Elewski, B. E. et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 78, 90–99.e1 (2018).
Castela, E. et al. Topical corticosteroids in plaque psoriasis: a systematic review of efficacy and treatment modalities. J. Eur. Acad. Dermatol. Venereol. 26, 36–46 (2012).
Hengge, U. R., Ruzicka, T., Schwartz, R. A. & Cork, M. J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 54, 1–15 (2006).
Kim, E. S. & Frampton, J. E. Calcipotriol/betamethasone dipropionate foam: a review in plaque psoriasis. Drugs 76, 1485–1492 (2016).
McCormack, P. L. Calcipotriol/betamethasone dipropionate: a review of its use in the treatment of psoriasis vulgaris of the trunk, limbs and scalp. Drugs 71, 709–730 (2011).
Sugarman, J. L. et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J. Drugs Dermatol. 16, 197–204 (2017).
Freeman, A. K. et al. Tacrolimus ointment for the treatment of psoriasis on the face and intertriginous areas. J. Am. Acad. Dermatol. 48, 564–568 (2003).
Kalb, R. E., Strober, B., Weinstein, G. & Lebwohl, M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J. Am. Acad. Dermatol. 60, 824–837 (2009).
Flytström, I., Stenberg, B., Svensson, A. & Bergbrant, I. M. Methotrexate vs. ciclosporin in psoriasis: effectiveness, quality of life and safety. A randomized controlled trial. Br. J. Dermatol. 158, 116–121 (2008).
Goldfarb, M. T. et al. Acitretin improves psoriasis in a dose-dependent fashion. J. Am. Acad. Dermatol. 18, 655–662 (1988).
Elmets, C. A. et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J. Am. Acad. Dermatol. 81, 775–804 (2019).
Archier, E. et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J. Eur. Acad. Dermatol. Venereol. 26, 11–21 (2012).
Hoy, S. M. & Scott, L. J. Etanercept: a review of its use in the management of ankylosing spondylitis and psoriatic arthritis. Drugs 67, 2609–2633 (2007).
Tobin, A.-M. & Kirby, B. TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs 19, 47–57 (2005).
Leonardi, C. L. et al. Etanercept as monotherapy in patients with psoriasis. N. Engl. J. Med. 349, 2014–2022 (2003).
Tyring, S. et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 367, 29–35 (2006).
Mease, P. J. et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356, 385–390 (2000).
Sterry, W. et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ 340, c147 (2010).
Antoni, C. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann. Rheum. Dis. 64, 1150–1157 (2005).
Gottlieb, A. B. et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J. Am. Acad. Dermatol. 51, 534–542 (2004).
Reich, K. et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366, 1367–1374 (2005).
Barker, J. et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br. J. Dermatol. 165, 1109–1117 (2011).
de Vries, A. C. et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the Psoriasis Infliximab vs. Etanercept Comparison Evaluation (PIECE) study. Br. J. Dermatol. 176, 624–633 (2017).
Menter, A. et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J. Am. Acad. Dermatol. 58, 106–115 (2008).
Gordon, K. et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J. Am. Acad. Dermatol. 66, 241–251 (2012).
Mease, P. et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N. Engl. J. Med. 377, 1537–1550 (2017).
McInnes, I. B. et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N. Engl. J. Med. 384, 1227–1239 (2021).
Kremenevski, I., Sander, O., Sticherling, M., Raithel, M. & LastName, F. M. Paradoxical reactions to biologicals in chronic inflammatory systemic diseases. Dtsch Arztebl Int. 119, 88–95 (2022).
Fania, L. et al. Paradoxical psoriasis induced by TNF-α blockade shows immunological features typical of the early phase of psoriasis development. J. Pathol. Clin. Res. 6, 55–68 (2020).
Krueger, J. G. et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J. Allergy Clin. Immunol. 144, 750–763 (2019).
Gordon, K. B. et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 375, 345–356 (2016).
McInnes, I. B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015).
Lebwohl, M. et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 373, 1318–1328 (2015).
Mease, P. J. et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 370, 2295–2306 (2014).
Mease, P. J., Helliwell, P. S., Hjuler, K. F., Raymond, K. & McInnes, I. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann. Rheum. Dis. 80, 185–193 (2021).
Gordon, K. B. et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 397, 475–486 (2021).
European medicines agency. Bimzelx: overview (European medicines agency, 2021).
Pharmaceuticals and Medical Devices Agency. New drugs approved in FY 2021 (Pharmaceuticals and Medical Devices Agency, 2022).
McInnes, I. B. et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 401, 25–37 (2023).
Reich, K. et al. Bimekizumab versus secukinumab in plaque psoriasis. N. Engl. J. Med. 385, 142–152 (2021).
Warren, R. B. et al. Bimekizumab versus adalimumab in plaque psoriasis. N. Engl. J. Med. 385, 130–141 (2021).
Yeilding, N. et al. Development of the IL-12/23 antagonist ustekinumab in psoriasis: past, present, and future perspectives. Ann. NY Acad. Sci. 1222, 30–39 (2011).
Kimball, A. B. et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: results from the PHOENIX 1 trial through up to 3 years. Br. J. Dermatol. 166, 861–872 (2012).
Papp, K. A. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 371, 1675–1684 (2008).
McInnes, I. B. et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 382, 780–789 (2013).
Gossec, L. et al. Persistence and effectiveness of the IL-12/23 pathway inhibitor ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: 1-year results from the real-world PsABio Study. Ann. Rheum. Dis. 81, 823–830 (2022).
Letarouilly, J.-G. et al. Secukinumab and ustekinumab treatment in psoriatic arthritis: results of a direct comparison. Rheumatology 60, 2773–2782 (2021).
Rich, P. et al. Ustekinumab improves nail disease in patients with moderate-to-severe psoriasis: results from PHOENIX 1. Br. J. Dermatol. 170, 398–407 (2014).
Gossec, L. et al. Long-term effectiveness and persistence of ustekinumab and TNF inhibitors in patients with psoriatic arthritis: final 3-year results from the PsABio real-world study. Ann. Rheum. Dis. 82, 496–506 (2022).
Puig, L., Morales-Múnera, C. E., López-Ferrer, A. & Geli, C. Ustekinumab treatment of TNF antagonist-induced paradoxical psoriasis flare in a patient with psoriatic arthritis: case report and review. Dermatology 225, 14–17 (2012).
Markham, A. Guselkumab: first global approval. Drugs 77, 1487–1492 (2017).
Deodhar, A. et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 395, 1115–1125 (2020).
McInnes, I. B. et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol. 73, 604–616 (2021).
Reich, K. et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 394, 831–839 (2019).
Reich, K. et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J. Am. Acad. Dermatol. 76, 418–431 (2017).
Papp, K. et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br. J. Dermatol. 173, 930–939 (2015).
Reich, K. et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 390, 276–288 (2017).
Menter, M. A. et al. The effect of tildrakizumab on cardiometabolic risk factors in psoriasis by metabolic syndrome status: post hoc analysis of two phase 3 trials (ReSURFACE 1 and ReSURFACE 2). J. Drugs Dermatol. 19, 703–708 (2020).
Wu, J. J. et al. Comparative cost-effectiveness of tildrakizumab and other commonly used treatments for moderate-to-severe psoriasis. J. Dermatol. Treat. 32, 693–700 (2021).
Jia, X. et al. Cost-effectiveness of tildrakizumab for the treatment of moderate-to-severe psoriasis in the United States. J. Dermatol. Treat. 33, 740–748 (2022).
Papp, K. A. et al. Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N. Engl. J. Med. 376, 1551–1560 (2017).
Gordon, K. B. et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 392, 650–661 (2018).
Reich, K. et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet 394, 576–586 (2019).
Bai, F. et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J. Immunol. Res. 2019, 2546161 (2019).
Iznardo, H. & Puig, L. IL-1 family cytokines in inflammatory dermatoses: pathogenetic role and potential therapeutic implications. Int. J. Mol. Sci. 23, 9479 (2022).
Furue, K. et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm. Venereol. 98, 5–13 (2018).
Bachelez, H. et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N. Engl. J. Med. 380, 981–983 (2019).
Bachelez, H. et al. Trial of spesolimab for generalized pustular psoriasis. N. Engl. J. Med. 385, 2431–2440 (2021).
Naik, H. B. et al. Anakinra for refractory pustular psoriasis: a phase II, open-label, dose-escalation trial. J. Am. Acad. Dermatol. 87, 1380–1383 (2022).
Tauber, M. et al. Partial clinical response to anakinra in severe palmoplantar pustular psoriasis. Br. J. Dermatol. 171, 646–649 (2014).
Chakraborty, A. et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1β monoclonal antibody. Clin. Pharmacokinet. 51, e1–e18 (2012).
Mansouri, B., Richards, L. & Menter, A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1β inhibitor gevokizumab. Br. J. Dermatol. 173, 239–241 (2015).
Boutet, M. A. et al. Interleukin-36 family dysregulation drives joint inflammation and therapy response in psoriatic arthritis. Rheumatology 59, 828–838 (2020).
Galluzzo, M. et al. Tofacitinib for the treatment of psoriasis. Expert Opin. Pharmacother. 17, 1421–1433 (2016).
Azevedo, A. & Torres, T. Tofacitinib: a new oral therapy for psoriasis. Clin. Drug Investig. 38, 101–112 (2018).
Berekmeri, A., Mahmood, F., Wittmann, M. & Helliwell, P. Tofacitinib for the treatment of psoriasis and psoriatic arthritis. Expert Rev. Clin. Immunol. 14, 719–730 (2018).
Mease, P. J. et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann. Rheum. Dis. 80, 312–320 (2021).
Zhang, J. et al. Application of baricitinib in dermatology. J. Inflamm. Res. 15, 1935–1941 (2022).
Słuczanowska-Głąbowska, S., Ziegler-Krawczyk, A., Szumilas, K. & Pawlik, A. Role of janus kinase inhibitors in therapy of psoriasis. J. Clin. Med. 10, 29–42 (2021).
Ciechanowicz, P., Rakowska, A., Sikora, M. & Rudnicka, L. JAK-inhibitors in dermatology: current evidence and future applications. J. Dermatol. Treat. 30, 648–658 (2019).
Gadina, M. et al. Translational and clinical advances in JAK-STAT biology: The present and future of jakinibs. J. Leukoc. Biol. 104, 499–514 (2018).
Chimalakonda, A. et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol. Ther. 11, 1763–1776 (2021).
Armstrong, A. W. et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J. Am. Acad. Dermatol. 88, 29–39 (2023).
Papp, K. et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med. 379, 1313–1321 (2018).
Tehlirian, C. et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J. Am. Acad. Dermatol. 87, 333–342 (2022).
Nogueira, M., Puig, L. & Torres, T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs 80, 341–352 (2020).
Li, G. et al. Advances in the development of phosphodiesterase-4 inhibitors. Eur. J. Med. Chem. 250, 115195 (2023).
Kavanaugh, A. et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann. Rheum. Dis. 73, 1020–1026 (2014).
Papp, K. et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J. Am. Acad. Dermatol. 73, 37–49 (2015).
Lebwohl, M. G. et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA 328, 1073–1084 (2022).
Pandya, V. B., Kumar, S., Sachchidanand, Sharma, R. & Desai, R. C. Combating autoimmune diseases with retinoic acid receptor-related orphan receptor-γ (RORγ or RORc) inhibitors: hits and misses. J. Med. Chem. 61, 10976–10995 (2018).
Imura, C. et al. A novel RORγt inhibitor is a potential therapeutic agent for the topical treatment of psoriasis with low risk of thymic aberrations. J. Dermatol Sci. 93, 176–185 (2019).
Hwang, S. et al. Interleukin-22 ameliorates neutrophil-driven nonalcoholic steatohepatitis through multiple targets. Hepatology 72, 412–429 (2020).
Barygina, V. et al. Fibroblasts to keratinocytes redox signaling: the possible role of ROS in psoriatic plaque formation. Antioxidants 8, 566 (2019).
Ouyang, W. & O’Garra, A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 50, 871–891 (2019).
Mahmud, M. R. et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14, 2096995 (2022).
Leijten, E. F. et al. Tissue-resident memory CD8+ T cells from skin differentiate psoriatic arthritis from psoriasis. Arthritis Rheumatol. 73, 1220–1232 (2021).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8, 726–736 (2008).
Galipeau, J. & Sensébé, L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22, 824–833 (2018).
Xu, F. et al. Mesenchymal stem cell-derived extracellular vesicles with high PD-L1 expression for autoimmune diseases treatment. Adv. Mater. 34, e2106265 (2022).
Riazifar, M. et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 13, 6670–6688 (2019).
Liu, R. et al. Mesenchymal stem cells in psoriatic lesions affect the skin microenvironment through circular RNA. Exp. Dermatol. 28, 292–299 (2019).
Hou, R. et al. Biological characteristics and gene expression pattern of bone marrow mesenchymal stem cells in patients with psoriasis. Exp. Dermatol. 23, 521–523 (2014).
Liang, N. et al. Dermal mesenchymal stem cells from psoriatic lesions stimulate HaCaT cell proliferation, differentiation, and migration via activating the PI3K/AKT signaling pathway. Dermatology 238, 283–291 (2022).
Campanati, A. et al. Indirect co-cultures of healthy mesenchymal stem cells restore the physiological phenotypical profile of psoriatic mesenchymal stem cells. Clin. Exp. Immunol. 193, 234–240 (2018).
Cheng, L. et al. Human umbilical cord mesenchymal stem cells for psoriasis: a phase 1/2a, single-arm study. Signal Transduct. Target Ther. 7, 263 (2022).
Tsuji, G. et al. Natural compounds tapinarof and galactomyces ferment filtrate downregulate IL-33 expression via the AHR/IL-37 axis in human keratinocytes. Front. Immunol. 13, 745997 (2022).
Altman, M. C. et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J. Clin. Invest. 129, 4979–4991 (2019).
Xu, M. et al. A UVB-responsive common variant at chromosome band 7p21.1 confers tanning response and melanoma risk via regulation of the aryl hydrocarbon receptor, AHR. Am. J. Hum. Genet. 108, 1611–1630 (2021).
Piccinni, M. P. et al. Medroxyprogesterone acetate decreases Th1, Th17, and increases Th22 responses via AHR signaling which could affect susceptibility to infections and inflammatory disease. Front. Immunol. 10, 642 (2019).
Holloman, B. L., Cannon, A., Wilson, K., Nagarkatti, P. & Nagarkatti, M. Aryl hydrocarbon receptor activation ameliorates acute respiratory distress syndrome through regulation of Th17 and Th22 cells in the lungs. mBio 14, e0313722 (2023).
Di Meglio, P. et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 40, 989–1001 (2014).
Bissonnette, R., Stein Gold, L., Rubenstein, D. S., Tallman, A. M. & Armstrong, A. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J. Am. Acad. Dermatol. 84, 1059–1067 (2021).
Jett, J. E. et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics. Am. J. Clin. Dermatol. 23, 83–91 (2022).
Lebwohl, M. G. et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N. Engl. J. Med. 385, 2219–2229 (2021).
Zhang, J., Cai, L. & Zheng, M. A novel topical treatment for plaque psoriasis: benvitimod/tapinarof. J. Am. Acad. Dermatol. 86, e137–e138 (2022).
Assaf, J., Sarkis, J. & Tomb, R. Reply to: Tapinarof cream 1% once daily and benvitimod 1% twice daily are two distinct topical medications. J. Am. Acad. Dermatol. 85, e203 (2021).
Ji, M. et al. Validating a selective S1P(1) receptor modulator Syl930 for psoriasis treatment. Biol. Pharm. Bull. 41, 592–596 (2018).
Liu, L. et al. Sphingosine-1-phosphate and its signal modulators alleviate psoriasis-like dermatitis: preclinical and clinical evidence and possible mechanisms. Front. Immunol. 12, 759276 (2021).
Masson Regnault, M., Shourick, J., Jendoubi, F., Tauber, M. & Paul, C. Time to relapse after discontinuing systemic treatment for psoriasis: a systematic review. Am. J. Clin. Dermatol. 23, 433–447 (2022).
Tian, D. & Lai, Y. The relapse of psoriasis: mechanisms and mysteries. JID Innov. 2, 100116 (2022).
Chen, L. & Shen, Z. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell Mol. Immunol. 17, 64–75 (2020).
Casey, K. A. et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012).
Matos, T. R. et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J. Clin. Invest. 127, 4031–4041 (2017).
Milner, J. J. & Goldrath, A. W. Transcriptional programming of tissue-resident memory CD8(+) T cells. Curr. Opin. Immunol. 51, 162–169 (2018).
Gallais Sérézal, I. et al. A skewed pool of resident T cells triggers psoriasis-associated tissue responses in never-lesional skin from patients with psoriasis. J. Allergy Clin. Immunol. 143, 1444–1454 (2019).
Puig, L. et al. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br. J. Dermatol. 186, 773–781 (2022).
Larsen, S. B. et al. Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell 28, 1758–1774.e1758 (2021).
Whitley, S. K. et al. Local IL-23 is required for proliferation and retention of skin-resident memory T(H)17 cells. Sci. Immunol. 7, eabq3254 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 81974478; 82173009) and Science Found for Creative Research Groups of the National Natural Science Foundation of China (82221002).
Author information
Authors and Affiliations
Contributions
XC and JS conceptualized the study and revised the manuscript. JG, HZ, and WL drafted the manuscript. LL checked the manuscript. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All patients provided written informed consent and study was approved by the institutional review boards (IRBs) of Xiangya Hospital, Central South University.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, J., Zhang, H., Lin, W. et al. Signaling pathways and targeted therapies for psoriasis. Sig Transduct Target Ther 8, 437 (2023). https://doi.org/10.1038/s41392-023-01655-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01655-6