Abstract
Background
The efficacy of pembrolizumab monotherapy in metastatic castration-resistant prostate cancer patients (mCRPC) when stratified by MSI-H and/or TMB-H is poorly defined. Additionally, outcomes based on sequencing source (i.e., tissue or liquid biopsy) have not been well described. We sought to assess outcomes of pembrolizumab monotherapy in patients with mCRPC and compare efficacy based on MSI-H and/or TMB-H when identified by tissue or liquid biopsy.
Methods
A retrospective analysis was performed of mCRPC patients treated at Mayo Clinic with pembrolizumab monotherapy between 2018 and 2023. Objective response rates (ORR), median progression-free survival (mPFS), and overall survival (mOS), were determined by RECIST v1.1 criteria.
Results
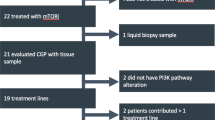
Twenty-two patients with mCRPC received pembrolizumab monotherapy for at least 3 cycles for a MSI-H or TMB-H indication. All patients had next generation sequencing (NGS) performed via tissue (n = 11) or liquid (n = 10) biopsy source. The ORR was 50% (27.3% complete response and 22.7% had partial response). The mPFS for TMB 10-14.9 mut/Mb (n = 4), TMB 15–24.9 mut/Mb (n = 6), and TMB ≥ 25 mut/Mb (n = 10) was 2.1, not reached (NR), and NR, respectively (p = 0.0003). The mOS for these same groups was 5.1 months, 20.5 months, and not reached, respectively. Among patients with TMB-H without co-occurring MSI-H or CDK12 (n = 6), none experienced a response and only one patient had stable disease compared to patients with MSI-H (n = 12) for whom the ORR was 75%. Immunotherapy responsive alterations such as ATRX and PTCH1 mutations were frequently noticed among patients who had complete response (CR).
Conclusions
Our hypothesis-generating study suggests that MSI-H drives the efficacy of pembrolizumab in mCRPC with better survival outcomes as TMB increases. Clinicians should consider alternative treatment strategies for advanced prostate cancer when TMB-H is present without co-occurring MSI-H or CDK12.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. The Lancet Oncol. 2020;21:1353–65.
Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N Engl J Med. 2017;377:1409–12.
Health, Center for Devices and Radiological. “List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools).” FDA, Aug. 2023. www.fda.gov, https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools.
Barata P, Agarwal N, Nussenzveig R, Gerendash B, Jaeger E, Hatton W, et al. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-H) detected by circulating tumor DNA. J Immunother Cancer. 2020;8:e001065.
Rauterkus G, Hadadi A, Barnett R, Weipert C, Drusbosky L, Gao X, et al. Blood-based tumor mutational burden from circulating tumor DNA and immune checkpoint inhibitors in advanced prostate cancer. Abstracts of the 2022 American Society of Clinical Oncology Meeting (ASC0). 2022;40:165.
Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non–small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. 2022;8:1160.
Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Annals Oncol. 2018;29:1807–13.
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. JCO. 2020;38:395–405.
Graf RP, Fisher V, Weberpals J, Gjoerup O, Tierno MB, Huang RSP, et al. Comparative effectiveness of immune checkpoint inhibitors vs chemotherapy by tumor mutational burden in metastatic castration-resistant prostate cancer. JAMA Netw Open. 2022;5:e225394.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. https://doi.org/10.1016/j.ejca.2016.03.081.
Lehner K, Ahmed ME, Bole R, Andrews JR, Haloi R, Bold MS, et al. High-volume mCRPC is associated with decreased cancer specific survival in patients on second-generation hormone therapy in the post docetaxel setting. J Clin Oncol. 2023;41:192–192.
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32:661–72.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34.
Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. JCO. 2016;34:1652–9.
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Human Pathol. 2000;31:578–83.
Taavitsainen S, Annala M, Ledet E, Beja K, Miller PJ, Moses M, et al. Evaluation of commercial circulating tumor DNA test in metastatic prostate cancer. JCO Precision Oncol. 2019;3:1–9.
Drusbosky L, Bilen MA, Azzi G, Barata PC, Boland PM, Bryce AH, et al. Blood-based tumor mutational burden from circulating tumor DNA (ctDNA) across advanced solid malignancies using a commercially available liquid biopsy assay. JCO. 2021;39:3040–3040.
Lin X, Wang L, Xie X, Qin Y, Xie Z, Ouyang M, et al. Prognostic biomarker TP53 mutations for immune checkpoint blockade therapy and its association with tumor microenvironment of lung adenocarcinoma. Front Mol Biosci. 2020;7:602328.
Sheng T, Li C, Zhang X, Chi S, He N, Chen K, et al. Activation of the Hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29.
Wang Y, Chen H, Jiao X, Wu L, Yang Y, Zhang J, et al. PTCH1 mutation promotes antitumor immunity and the response to immune checkpoint inhibitors in colorectal cancer patients. Cancer Immunol Immunother. 2022;71:111–20.
Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17.
Ge Y, Wang Z, Li H, Liu Y, Wei P. Association of ATRX mutations with immunologically active characteristics in patients with MSI-prone tumors. Am J Transl Res. 2022;14:6107–22.
Jacob A, Raj R, Allison DB, Myint ZW. Androgen receptor signaling in prostate cancer and therapeutic strategies. Cancers. 2021;13:5417.
Antonarakis ES, Isaacsson Velho P, Fu W, Wang H, Agarwal N, Santos VS, et al. “CDK12 -altered prostate cancer: clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-Ribose) polymerase inhibitors, and PD-1 inhibitors.” JCO Precision Oncol. 2020;4:370–81.
Sturgill EG, Misch A, Jones CC, Luckett D, Fu X, Schlauch D, et al. Discordance in tumor mutation burden from blood and tissue affects association with response to immune checkpoint inhibition in real-world settings. The Oncologist. 2022;3:175–82.
Author information
Authors and Affiliations
Contributions
Protocol / project development: OM, AK. Data collection or management: OM, AK. Data analysis: OM, AK. Manuscript writing / editing: OM, AK, JO, WT. Other (Interpretation of data and revision): OM, WT, AB, RD, DC, LP, JO, AK.
Corresponding author
Ethics declarations
Competing interests
LCP; research support from Exelixis. DSC; Honoria from Targeted Oncology, IntrinsiQ MJH Life Sciences, International Centers for Precision Oncology Foundation. Consulting or advisory for Janssen Biotech and Novartis.
Ethics approval
Institutional review board approval IRB: 19-009479. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mosalem, O., Tan, W., Bryce, A.H. et al. A real-world experience of pembrolizumab monotherapy in microsatellite instability-high and/or tumor mutation burden-high metastatic castration-resistant prostate cancer: outcome analysis. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00799-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00799-y