Abstract
Background
Recent studies have highlighted the association between androgen deprivation therapy (ADT) and the gut microbiota in prostate cancer. However, the impact of long-term ADT remains to be explored.
Methods
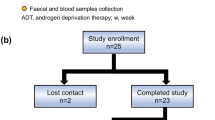
To examine changes in the gut microbial profile from short-term (a median of 7 months), and middle-term (a median of 18 months) to long-term ADT (>33 months), 16S rRNA data from 56 fecal samples were reanalyzed. Additionally, a two-sample Mendelian randomization was employed to investigate the relationships between particular microbial signatures and prostate cancer as well as testosterone levels.
Results
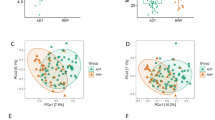
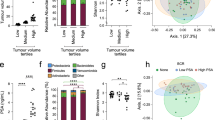
In contrast to the short- and middle-term ADT groups, the long-term ADT group had significant changes in alpha and beta diversity. In particular, the relative abundance of genera such as Catenibacterium and Holdemanella decreased in the long-term ADT group, whereas the opportunistic bacterium (Erysipelatoclostridium) and Ruminococcus gnavus showed increased abundance over ADT time. Moreover, a two-sample Mendelian randomization analysis revealed the negative associations between genetically predicated genera Coprobacter, Ruminococcaceae UCG002/011, and Defluviitaleacea-UCG-011, and testosterone levels.
Conclusions
In conclusion, long-term ADT use in prostate cancer patients was associated with detrimental changes in gut microbiota, including an increase in genera related to testosterone synthesis and opportunistic bacteria. These changes may be related to disease progression and side effects of long-term ADT while further longitudinal studies are required to prove this relationship.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The 16S rRNA-seq was downloaded from the accession number PRJNA690135 [6]. The in-house R scripts used to perform microbial analysis by the “microeco” R package [37] and generate figures are available on GitHub (https://github.com/lynnLW/long-term-ADT).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49.
Zhu Y, Mo M, Wei Y, Wu J, Pan J, Freedland SJ, et al. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol. 2021;18:282–301.
Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44.
Chandrasekar T, Yang JC, Gao AC, Evans CP. Mechanisms of resistance in castration-resistant prostate cancer (CRPC). Transl Androl Urol. 2015;4:365–80.
Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–11.
Li JKM, Wang LL, Wong CYP, Chiu PKF, Teoh JYC, Kwok HSW, et al. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2021;24:1063–72.
Markowski MC, Sfanos KS. The interplay of microbiota and hormone regulation in men with prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:935–6.
Sfanos KS, Markowski MC, Peiffer LB, Ernst SE, White JR, Pienta KJ, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21:539–48.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Terrisse S, Goubet AG, Ueda K, Thomas AM, Quiniou V, Thelemaque C, et al. Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J Immunother Cancer. 2022;10:e004191.
Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Calì B, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. 2021;374:216–24.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020:2020.08.10.244293. https://doi.org/10.1101/2020.08.10.244293.
Anderson MJ. Permutational multivariate analysis of variance. Department of Statistics, Univerisity of Auckland. 2005;26:32–46.
Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–8.
Morgans AK, Fan KH, Koyama T, Albertsen PC, Goodman M, Hamilton AS, et al. Bone complications among prostate cancer survivors: long-term follow-up from the prostate cancer outcomes study. Prostate Cancer Prostatic Dis. 2014;17:338–42.
Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao J, Petrosino JF, et al. Gut microbiome associates with lifetime cardiovascular disease risk profile among bogalusa heart study participants. Circ Res. 2016;119:956–64.
Kure A, Tsukimi T, Ishii C, Aw W, Obana N, Nakato G, et al. Gut environment changes due to androgen deprivation therapy in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2023;26:323–30.
Zhong W, Wu K, Long Z, Zhou X, Zhong C, Wang S, et al. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome. 2022;10:94.
Fujita K, Matsushita M, Banno E, De Velasco MA, Hatano K, Nonomura N, et al. Gut microbiome and prostate cancer. Int J Urol. 2022;29:793–8.
Martins CDA, Rocha GDG, Gattass CR, Takiya CM. Pomolic acid exhibits anticancer potential against a docetaxel‑resistant PC3 prostate cell line. Oncol Rep. 2019;42:328–38.
Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H, et al. Gut microbiota–derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81:4014–26.
Ma Y, Zhu L, Ma Z, Gao Z, Wei Y, Shen Y, et al. Distinguishing feature of gut microbiota in Tibetan highland coronary artery disease patients and its link with diet. Sci Rep. 2021;11:18486.
Zapatero A, Guerrero A, Maldonado X, Alvarez A, Segundo CGS, Rodríguez MAC, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320–7.
Jain S, Samal AG, Das B, Pradhan B, Sahu N, Mohapatra D, et al. Escherichia coli, a common constituent of benign prostate hyperplasia-associated microbiota induces inflammation and DNA damage in prostate epithelial cells. Prostate. 2020;80:1341–52.
Tsai KY, Wu DC, Wu WJ, Wang JW, Juan YS, Li CC, et al. Exploring the association between gut and urine microbiota and prostatic disease including benign prostatic hyperplasia and prostate cancer using 16S rRNA sequencing. Biomedicines. 2022;10:2676.
Li L-Y, Han J, Wu L, Fang C, Li W-G, Gu J-M, et al. Alterations of gut microbiota diversity, composition and metabonomics in testosterone-induced benign prostatic hyperplasia rats. Military Med Res. 2022;9:12.
Liu X, Tang H, Zhou Q, Zeng Y, Lu B, Chen D, et al. Gut microbiota composition in patients with advanced malignancies experiencing immune-related adverse events. Front Immunol. 2023;14:1109281.
Cai S, Yang Y, Kong Y, Guo Q, Xu Y, Xing P, et al. Gut Bacteria Erysipelatoclostridium and Its Related Metabolite Ptilosteroid A Could Predict Radiation-Induced Intestinal Injury. Front Public Health. 2022;10:862598.
Freier TA, Beitz DC, Li L, Hartman PA. Characterization of eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe†. Int J Syst Evol Microbiol. 1994;44:137–42.
Miller WL. Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol. 2002;198:7–14.
Mohiuddin JJ, Baker BR, Chen RC. Radiotherapy for high-risk prostate cancer. Nat Rev Urol. 2015;12:145–54.
Mjaess G, Karam A, Roumeguère T, Diamand R, Aoun F, McVary K, et al. Urinary microbiota and prostatic diseases: the key for the lock? A systematic review. Prostate Cancer Prostatic Dis. 2022;26:451–60.
Lombardo R, Tema G, Cornu JN, Fusco F, McVary K, Tubaro A, et al. The urothelium, the urinary microbioma and men LUTS: a systematic review. Minerva Urol Nefrol. 2020;72:712–22.
Kwa WT, Sundarajoo S, Toh KY, Lee J. Application of emerging technologies for gut microbiome research. Singapore Med J. 2023;64:45–52.
Liu C, Cui Y, Li X, Yao M. microeco: an R package for data mining in microbial community ecology. FEMS Microbiol Ecol. 2021;97:fiaa255.
Acknowledgements
Summary-level GWAS data for prostate cancer were obtained from the UK Biobank study (Neale Lab) and the FinnGen consortium. The 16S rRNA-seq data were obtained from the NCBI website which was uploaded by Prof. NG Chi Fai’s lab. We thank all investigators for sharing their public data.
Funding
The work was supported by grants from the Scientific Research Initiation Fund Project of Zhengzhou Central Hospital (KYQDJJ2023004).
Author information
Authors and Affiliations
Contributions
All contributions were from WL.
Corresponding author
Ethics declarations
Competing interests
The author declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L. Changes in the gut microbial profile during long-term androgen deprivation therapy for prostate cancer. Prostate Cancer Prostatic Dis (2023). https://doi.org/10.1038/s41391-023-00723-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-023-00723-w