Abstract
Background
In patients presenting with metastatic prostate cancer, the role of local therapy is evolving. Two recently reported large-scale randomized trials suggest that radiotherapy (RT) directed at the prostate improves overall survival (OS) in patients with low metastatic burden. We reviewed the experience of prostate RT in this setting at our center.
Methods
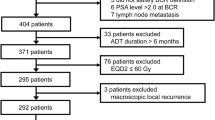
The study population consisted of men with newly-diagnosed metastatic hormone-sensitive prostate cancer (mHSPC) referred to a comprehensive cancer center between 2005 and 2015 and treated initially with androgen deprivation therapy. Patients were eligible for inclusion if they received (1) prostate RT with biological effective dose (BED) at least that of a course of 40 Gy in 15 fractions or (2) no prostate RT. The association between receipt of prostate RT and OS was studied. OS was estimated using the Kaplan–Meier method and Cox regression was used to identify factors associated with OS.
Results
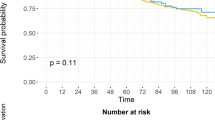
The cohort consisted of 410 patients, of whom 128 received prostate RT. Median follow-up 61.0 months. On univariate analysis, receipt of prostate RT was associated with improved OS (HR 0.59, 95% CI 0.45–0.77, p = 0.0001). Median OS in those patients receiving prostate RT was 47.4 months versus 26.3 months in those not receiving prostate RT. In a multivariate Cox model, receipt of prostate RT remained associated with improved OS (HR 0.69, 95% CI 0.50–0.94, p = 0.02). In those treated with prostate RT, increasing BED was also associated with improved OS (HR 0.87 per 10 Gy increase, 95% CI 0.76–0.99, p = 0.03).
Conclusions
This cohort represents the largest single-center experience of primary tumor-directed RT in mHSPC reported to date. In this population, receipt of prostate RT was associated with improved OS and the magnitude of the OS benefit was clinically significant. The possibility of an RT dose-response gradient in this setting merits further study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65(Jun):1058–66. https://doi.org/10.1016/j.eururo.2013.11.012.
Satkunasivam R, Kim AE, Desai M, Nguyen MM, Quinn DI, Ballas L, et al. Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: a SEER-medicare analysis. J Urol. 2015;194(Aug):378–85. https://doi.org/10.1016/j.juro.2015.02.084.
Rusthoven CG, Jones BL, Flaig TW, Crawford ED, Koshy M, Sher DJ, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34:2835–42. https://doi.org/10.1200/JCO.2016.67.4788. 08.
Morgan SC, Parker CC. Local treatment of metastatic cancer–killing the seed or disturbing the soil?. Nat Rev Clin Oncol. 2011;8(Jun):504–6. https://doi.org/10.1038/nrclinonc.2011.88.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–66. https://doi.org/10.1016/s0140-6736(18)32486-3.
Boeve LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75(Mar):410–8. https://doi.org/10.1016/j.eururo.2018.09.008.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(Mar):1148–59. https://doi.org/10.1200/JCO.2007.12.4487.
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. (Eds.) AJCC Cancer Staging Manual. 8 ed. Chicago: Springer International Publishing; 2017. p.1032.
Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340(Mar):b5087 https://doi.org/10.1136/bmj.b5087.
Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl J Med. 2016;375(Oct):1425–37. https://doi.org/10.1056/NEJMoa1606221.
Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl J Med. 2008;358(Mar):1250–61. https://doi.org/10.1056/NEJMoa074311.
Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLoS Clin Trials. 2006;1(May):e9 https://doi.org/10.1371/journal.pctr.0010009.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl J Med. 2015;373(Aug):737–46.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(Mar):1163–77.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2017;377(Jul):352–60.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl J Med. 2017;377(Jul):338–51.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S. et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl J Med. 2019;381(Jul):121–31. https://doi.org/10.1056/NEJMoa1903835.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2019;381(Jul):13–24. https://doi.org/10.1056/NEJMoa1903307.
Author information
Authors and Affiliations
Contributions
Conception and design: SCM, SM, OEH. Administrative support: SG, JC. Provision of study material or patients: SM, SCM. Collection and assembly of data: JC, SG, SCM. Data analysis and interpretation: SCM, SM, OEH. Initial paper writing: SCM. Critical review and final approval of paper: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests in relation to this work. Outside of this work, SCM has served in a consulting or advisory role for Astellas Pharma, Bayer, Janssen, and Tersera. Outside of this work, SM has received honoraria from Astellas Pharma, Bayer, Janssen, and Sanofi, and has received travel and accommodation support from Sanofi and Tersera. The other authors have no financial disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morgan, S.C., Holmes, O.E., Craig, J. et al. Long-term outcomes of prostate radiotherapy for newly-diagnosed metastatic prostate cancer. Prostate Cancer Prostatic Dis 24, 1041–1047 (2021). https://doi.org/10.1038/s41391-021-00339-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00339-y
This article is cited by
-
Health-related quality of life of metastatic prostate cancer patients treated with prostate Radiotherapy
BMC Cancer (2023)
-
Importance of radiotherapy to the primary in metastatic hormone sensitive prostate cancer
Prostate Cancer and Prostatic Diseases (2021)