Abstract
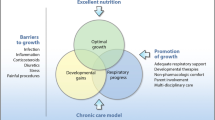
Chronic lung disease of prematurity or bronchopulmonary dysplasia (BPD) is a common complication of preterm birth. Nutrition may affect incidence and severity of BPD. In this context, the Section on Nutrition, Gastroenterology and Metabolism, the Pulmonary Section of the European Society for Paediatric Research (ESPR) and SPR have joined forces to review the current knowledge on nutritional issues related to BPD. The aim of this narrative review is to discuss the clinical implications for nutritional practice. Nutrient deficiencies may influence pathogenesis of BPD. Adequate nutrition and growth can play a crucial role in the prevention of and recovery from BPD. Optimal nutrition strategy is an important principle, especially in the early postnatal period. As optimal energy intake in infants at risk of BPD or with evolving BPD is not yet defined, further research with well-designed studies on nutritional strategies for preterm infants with BPD is urgently needed.
Impact
-
Based on current evidence it seems reasonable to recommend that BPD diagnosed infants should receive an energy supply ranging from 120 to 150 Kcal/kg/d.
-
Exclusive MOM feed with adequate fortification should be encouraged as this is associated with a significant reduction in the risk of BPD.
-
Suboptimal nutritional delivery is often seen in preterm infants with BPD compared to controls.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Jobe, A. H. Mechanisms of lung injury and bronchopulmonary dysplasia. Am. J. Perinatol. 33, 1076–1078 (2016).
Paananen, R. et al. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J. Pediatr. 154, 39–43e3 (2009).
Heras, A., Chambers, R., Solomon, Z., Blatt, L. & Martin, C. R. Nutrition-based implications and therapeutics in the development and recovery of bronchopulmonary dysplasia. Semin Perinatol. 47, 1518182023 (2023).
Correani, A. et al. Reduced pulmonary oxygen diffusion at 36 weeks of postmenstrual age in small-for-gestational-age preterm infants of less than 32 weeks without bronchopulmonary dysplasia. Pediatr. Pulmonol. 58, 3054–3062 (2023).
Morrow, L. A. et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am. J. Respir. Crit. Care Med. 196, 364–374 (2017).
Bose, C. et al. Extremely low gestational age newborn study investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124, e450–e458 (2009).
Polin, R., Abman, S.H., Rowitch, D.H., Benitz, W. Fetal and Neonatal Physiology. 6th edn. (Elsevier, Philadelphia, 2022).
Ehrenkranz, R. A. et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 117, 1253–1261 (2006).
Frank, L. & Sosenko, I. R. Undernutrition as a major contributing factor in the pathogenesis of bronchopulmonary dysplasia. Am. Rev. Respir. Dis. 138, 725–729 (1988).
Poindexter, B. B. & Martin, C. R. Impact of nutrition on bronchopulmonary dysplasia. Clin. Perinatol. 42, 797–806 (2015).
Uberos, J., Lardón-Fernández, M., Machado-Casas, I., Molina-Oya, M. & Narbona-López, E. Nutrition in extremely low birth weight infants: Impact on bronchopulmonary dysplasia. Minerva Paediatr. 68, 419–426 (2016).
Thiess, T. et al. Correlation of early nutritional supply and development of bronchopulmonary dysplasia in preterm infants <1000 g. Front Pediatr. 9, 741365 (2021).
Klevebro, S. et al. Early energy and protein intakes and associations with growth, BPD, and ROP in extremely preterm infants. Clin. Nutr. 38, 1289–1295 (2019).
Atkinson, S. A. Special nutritional needs of infants for prevention of and recovery from bronchopulmonary dysplasia. J. Nutr. 131, 942S–946S (2001).
Arigliani, M., Spinelli, A. M., Liguoro, I. & Cogo, P. Nutrition and lung growth. Nutrients 10, 919 (2018).
Oh, W. et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 147, 786–790 (2005).
Rocha, G., Guimarães, H. & Pereira-da-Silva, L. The role of nutrition in the prevention and management of bronchopulmonary dysplasia: a literature review and clinical approach. Int J. Environ. Res Public Health 18, 6245 (2021).
Principi, N., Di Pietro, G. M. & Esposito, S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J. Transl. Med. 16, 1–13 (2018).
Embleton, N. D. et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. J. Pediatr. Gastroenterol. Nutr. 76, 248–268 (2023).
Johnson, M. J. et al. Research priorities in pediatric parenteral nutrition: a consensus and perspective from ESPGHAN/ESPEN/ESPR/CSPEN. Pediatr. Res. 92, 61–70 (2022).
Hamosh, M. Lipid metabolism in pediatric nutrition. Pediatr. Clin. North Am. 42, 839–859 (1995).
Giretti, I. et al. Hypertriglyceridemia and lipid tolerance in preterm infants with a birth weight of less than 1250 g on routine parenteral nutrition. Clin. Nutr. 40, 4444–4448 (2021).
Biagetti, C. et al. Double blind exploratory study on de novo lipogenesis in preterm infants on parenteral nutrition with a lipid emulsion containing 10% fish oil. Clin. Nutr. 35, 337–343 (2016).
Correani, A. et al. Oxygen saturation to fraction of inspired oxygen ratio in preterm infants on routine parenteral nutrition with conventional or fish oil containing lipid emulsions. Pediatr. Pulmonol. 55, 2377–2382 (2020).
Collins, C. T. et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N. Engl. J. Med 376, 1245–1255 (2017).
Spiegler, J. et al. Does breastmilk influence the development of bronchopulmonary dysplasia? J. Pediatr. 169, 76–80.e4 (2016).
Villamor-Martínez, E., Pierro, M., Cavallaro, G., Mosca, F. & Villamor, E. Mother’s own milk and bronchopulmonary dysplasia: a systematic review and meta-analysis. Front Pediatr. 7, 224 (2019).
Huang, J. et al. Human milk as a protective factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 104, F128–F136 (2019).
Xu, Y. et al. Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatr. 20, 522 (2020).
Fonseca, L. T., Senna, D. C., Silveira, R. C. & Procianoy, R. S. Association between breast milk and bronchopulmonary dysplasia: a single center observational study. Am. J. Perinatol. 7, 264–269 (2017).
Biniwale, M. A. & Ehrenkranz, R. A. The role of nutrition in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 30, 200–208 (2006).
Bauer, J., Maier, K., Muehlbauer, B., Poeschl, J. & Linderkamp, O. Energy expenditure and plasma catecholamines in preterm infants with mild chronic lung disease. Early Hum. Dev. 72, 147–157 (2003).
Lima, P. A. T. et al. Energy expenditure and body composition in infants with bronchopulmonary dysplasia at term age. Eur. J. Pediatr. 181, 3039–3047 (2022).
Bauer, S. E., Huff, K. A., Vanderpool, C. P. B., Rose, R. S. & Cristea, A. I. Growth and nutrition in children with established bronchopulmonary dysplasia: a review of the literature. Nutr. Clin. Pr. 37, 282–298 (2022).
Giannì, M. L. et al. The role of nutrition in promoting growth in pre-term infants with bronchopulmonary dysplasia: a prospective non-randomised interventional cohort study. BMC Pediatr. 14, 235 (2014).
Miller, A. N., Curtiss, J., Taylor, S. N., Backes, C. H. & Kielt, M. J. A review and guide to nutritional care of the infant with established bronchopulmonary dysplasia. J. Perinat. 43, 402–410 (2023).
Jensen, E. A. et al. Individualising care in severe bronchopulmonary dysplasia: a series of N-of-1 trials comparing transpyloric and gastric feeding. Arch. Dis. Child Fetal Neonatal Ed. 105, 399–404 (2020).
Crawford, M. A. et al. The imperative of arachidonic acid in human reproduction. Prog. Lipid Res. 91, 101222 (2023).
Lapillonne, A., Eleni dit Trolli, S. & Kermorvant-Duchemin, E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology 98, 397–403 (2010).
Leuti, A., Maccarrone, M. & Chiurchiù, V. Proresolving lipid mediators: endogenous modulators of oxidative stress. Oxid. Med Cell Longev. 18, 8107265 (2019).
Martin, C. R. et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 159, 743–749e1-2 (2011).
Manley, B. J. et al. High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics 128, e71–e77 (2011).
Marc, I. et al. Effect of maternal docosahexaenoic acid supplementation on bronchopulmonary dysplasia-free survival in breastfed preterm infants: a randomized clinical trial. JAMA 324, 157–167 (2020).
Wendel, K. et al. Effect of arachidonic and docosahexaenoic acid supplementation on respiratory outcomes and neonatal morbidities in preterm infants. Clin. Nut 42, 22–28 (2023).
Ryan, A. & Godson, C. Lipoxins: regulators of resolution. Curr. Opin. Pharm. 10, 166–172 (2010).
Martin, C. R. et al. Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia-induced lung injury. PLoS One 9, e98773 (2014).
Perrotta, S. et al. Vitamin A and infancy. Biochemical, functional, and clinical aspects. Vitam. Hormones 66, 457–591 (2003).
Biesalski, H. K. & Nohr, D. Importance of vitamin-A for lung function and development. Mol. Asp. Med. 24, 431–440 (2003).
Massaro, D. & Massaro, G. D. Lung development, lung function, and retinoids. N. Engl. J. Med. 362, 1829–1831 (2010).
Shenai, J. P. Vitamin A supplementation in very low birth weight neonates: rationale and evidence. Pediatrics 104, 1369–1374 (1999).
Blaner, W. S., Shmarakov, I. O. & Traber, M. G. Vitamin A and Vitamin E: will the real antioxidant please stand up? Annu Rev. Nutr. 41, 105–131 (2021).
Agostoni, C. et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 50, 85–91 (2010).
Rossholt, M. E. et al. Vitamin A status in preterm infants is associated with inflammation and dexamethasone exposure. Nutrients 15, 441 (2023).
Darlow, B. A., Graham, P. J. & Rojas-Reyes, M. X. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst. Rev. 22, CD000501 (2016).
Mactier, H. Vitamin A for preterm infants; where are we now? Semin. Fetal Neonatal Med. 18, 166–171 (2013).
Inder, T. E., Graham, P. J., Winterbourn, C. C., Austin, N. C. & Darlow, B. A. Plasma vitamin A levels in the very low birthweight infant-relationship to respiratory outcome. Early Hum. Dev. 52, 155–168 (1998).
Spears, K., Cheney, C. & Zerzan, J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long-term respiratory disability in very-low-birth-weight infants. Am. J. Clin. Nutr. 80, 1589–1594 (2004).
Phattraprayoon, N., Ungtrakul, T., Soonklang, K. & Susantitaphong, P. Oral vitamin A supplementation in preterm infants to improve health outcomes: A systematic review and meta-analysis. PLoS One 17, e0265876 (2022).
Rakshasbhuvankar, A. A., Pillow, J. J., Simmer, K. N. & Patole, S. K. Vitamin A supplementation in very-preterm or very-low-birth-weight infants to prevent morbidity and mortality: a systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 114, 2084–2096 (2021).
Rakshasbhuvankar, A. A. et al. Enteral Vitamin A for reducing severity of bronchopulmonary dysplasia: a randomized trial. Pediatrics 147, e2020009985 (2021).
Basu, S., Khanna, P., Srivastava, R. & Kumar, A. Oral vitamin A supplementation in very low birth weight neonates: a randomized controlled trial. Eur. J. Pediatr. 178, 1255–1265 (2019).
Sun, H., Cheng, R. & Wang, Z. Early vitamin a supplementation improves the outcome of retinopathy of prematurity in extremely preterm infants. Retina 40, 1176–1184 (2020).
Majerus, P. W. Inositol phosphate biochemistry. Annu Rev. Biochem. 61, 225–250 (1992).
Chatree, S., Thongmaen, N., Tantivejkul, K., Sitticharoon, C. & Vucenik, I. Role of inositols and inositol phosphates in energy metabolism. Molecules 25, 5079 (2020).
Russo, M., Forte, G., Montanino Oliva, M., Laganà, A. S. & Unfer, V. Melatonin and myo-inositol: supporting reproduction from the oocyte to birth. Int J. Mol. Sci. 22, 8433 (2021).
Hallman, M. & Epstein, B. L. Role of myo-inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Biochem Biophys. Res Commun. 92, 1151–1159 (1980).
Guarner, V., Tordet, C. & Bourbon, J. R. Effects of maternal protein-calorie malnutrition on the phospholipid composition of surfactant isolated from fetal and neonatal rat lungs. Compensation by inositol and lipid supplementation. Pediatr. Res 31, 629–635 (1992).
Bromberger, P. & Hallman, M. Myoinositol in small preterm infants: relationship between intake and serum concentration. J. Pediatr. Gastroenterol. Nutr. 5, 455–458 (1986).
Brion, L. P. et al. Blood myo-inositol concentrations in preterm and term infants. J. Perinatol. 41, 247–254 (2021).
Nilsson, A. K. et al. Longitudinal serum metabolomics in extremely premature infants: relationships with gestational age, nutrition, and morbidities. Front Neurosci. 16, 830884 (2022).
Hallman, M., Arjomaa, P. & Hoppu, K. Inositol supplementation in respiratory distress syndrome: relationship between serum concentration, renal excretion, and lung effluent phospholipids. J. Pediatr. 110, 604–610 (1987).
Phelps, D. L. et al. Safety and pharmacokinetics of multiple dose myo-inositol in preterm infants. Pediatr. Res 80, 209–217 (2016).
Hallman, M., Järvenpää, A. L. & Pohjavuori, M. Respiratory distress syndrome and inositol supplementation in preterm infants. Arch. Dis. Child 61, 1076–1083 (1986).
Hallman, M., Bry, K., Hoppu, K., Lappi, M. & Pohjavuori, M. Inositol supplementation in premature infants with respiratory distress syndrome. N. Engl. J. Med 326, 1233–1239 (1992).
Friedman, C. A. et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. J. Pediatr. Ophthalmol. Strabismus 37, 79–86 (2000).
Phelps, D. L. et al. Effects of Myo-inositol on Type 1 retinopathy of prematurity among preterm infants <28 Weeks’ gestational age: a randomized clinical trial. JAMA 320, 1649–1658 (2018).
Howlett, A., Ohlsson, A. & Plakkal, N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst. Rev. 7, CD000366 (2019).
Lingappan, K., Arunachalam, A. & Pammi, M. Lactoferrin and the newborn: current perspectives. Expert Rev. Anti Infect. Ther. 11, 695–707 (2013).
Faust, K. B. et al. Lactoferrin and human neutrophil protein (HNP) 1-3 levels during the neonatal period in preterm infants. Front Pediatr. 10, 909176 (2022).
Revenis, M. E. & Kaliner, M. A. Lactoferrin and lysozyme deficiency in airway secretions: association with the development of bronchopulmonary dysplasia. J. Pediatr. 121, 262–270 (1992).
Gao, Y. et al. Enteral lactoferrin supplementation for preventing sepsis and necrotizing enterocolitis in preterm infants: a meta‑analysis with trial sequential analysis of randomized controlled trials. Front Pharm. 11, 11862020 (2020).
Qu, Y., Guo, S., Liu, Y., Wang, G. & Wu, H. Association between probiotics and bronchopulmonary dysplasia in preterm infants. Sci. Rep. 11, 17060 (2021).
Funding
No financial assistance was received in support of the study.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study’s conception and design. Literature search performed in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and the Cochrane Library (http://www.cochranelibrary.com/) were performed by A.G. Each section was written by a separate author. Leif D. Nelin, Ana Sánchez, and Minesh Khashu provided important scientific contribution. Miguel Saenz de Pipaon drafted the manuscript. All authors commented on previous versions of the manuscript and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All the members of the ESPR Nutrition council members reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saenz de Pipaon, M., Nelin, L.D., Gehred, A. et al. The role of nutritional interventions in the prevention and treatment of chronic lung disease of prematurity. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03133-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03133-3
This article is cited by
-
Adequate nutrition for bronchopulmonary dysplasia, but do not forget oxygen
Pediatric Research (2024)