Abstract
Objective
To evaluate the accuracy of diagnostic algorithms developed using the International Classification of Diseases (ICD-9-CM and ICD-10-CA) diagnostic codes and physician billing codes for thromboembolism (TE) from health administrative data compared to chart review diagnoses of TE in children with cancer.
Methods
Using data linkage between the Pediatric Oncology Group of Ontario Network Information System (Ontario pediatric cancer registry) and various administrative data housed at ICES, eight algorithms were developed including a single reference to one of the billing codes, multiple references with varying time intervals, and combinations of various billing codes during primary cancer therapy for the whole cohort and, for early (<04/2002) and later (≥04/2002, solely ICD-10 codes) periods. Reference standard was chart review data from prior studies (from 1990 to 2016) among children (≤19 years) with cancer and radiologically confirmed TE.
Results
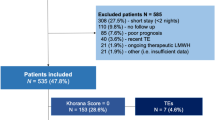
Records of 2056 patients diagnosed with cancer at two participating sites during study period were reviewed; 112 had radiologically confirmed TE. The algorithm with addition of anticoagulation utilization codes was the best performing algorithm (sensitivity = 0.76;specificity = 0.85). With use of ICD-10 only codes, sensitivity of the same algorithm improved to 0.84 with specificity of 0.80.
Conclusion
This study provides a valid approach for ascertaining pediatric TE using real-world data.
Impact
-
Research in pediatric thrombosis, especially cancer-related thrombosis, is limited mainly due to small-sized studies.
-
Real-world data provide ready access to large and diverse populations. However, there are no validated algorithms for identifying thrombosis in real-world data for children.
-
An algorithm based on combination of thrombosis and anticoagulation utilization codes had 76% sensitivity and 85% specificity to identify diagnosis of thrombosis in children in administrative data.
-
This study provides a valid approach for ascertaining pediatric thrombosis using real-world data and offers a good avenue to advance pediatric thrombosis research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
References
Raffini, L., Huang, Y.-S., Witmer, C. & Feudtner, C. Dramatic increase in venous thromboembolism in U.S. Children’s Hospitals from 2001–2007. Pediatrics 124, 1001–1008 (2009).
O’Brien, S. H., Klima, J., Termuhlen, A. M. & Kelleher, K. J. Venous thromboembolism and adolescent and young adult oncology inpatients in US Children’s Hospitals, 2001 to 2008. J. Pediatr. 159, 133–137 (2011).
Pelland-Marcotte, M.-C. et al. Thromboembolism incidence and risk factors in children with cancer: a population-based cohort study. Thromb. Haemost. 118, 1646–1655 (2018).
Athale, U. Thrombosis in pediatric cancer: identifying the risk factors to improve care. Expert Rev. Hematol. 6, 599–609 (2013).
Ranta, S. et al. Cerebro-sinovenous thrombosis in children with acute lymphoblastic leukemia- a multicenter study from nordic pediatric hematology oncology. Br. J. Haematol. 168, 547–552 (2015).
Tuckuviene, R. et al. Prospective study of thromboembolism in 1038 children with acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology (NOPHO) study. J. Thromb. Haemost. 14, 485–494 (2016).
Caruso, V. et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 patients. Blood 108, 2216–2222 (2006).
Mitchell, L. G. et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase. Results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) study. Cancer 97, 508–516 (2003).
Paz-Priel, I., Long, L., Helman, L. J., Mackall, C. L. & Wayne, A. S. Thromboembolic events in children and young adults with pediatric sarcoma. J. Clin. Oncol. 25, 1519–1524 (2007).
Grace, R. F. et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br. J. Haematol. 152, 452–459 (2011).
Athale, U. et al. Predictors of thrombosis in children receiving therapy for acute lymphoblastic leukemia: results from Dana-Farber Cancer Institute ALL Consortium trial 05-001. Pediatr. Blood Cancer 69, e29581 (2022).
Setty, B. A., O’Brien, S. H. & Kerlin, B. A. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr. Blood Cancer 59, 258–264 (2012).
Forbrigger, Z. et al. The association of venous thromboembolism with survival in pediatric cancer patients: a population-based cohort study. Ann. Hematol. 97, 1903–1908 (2018).
Kulkarni, K. et al. Increased requirement for central venous catheter replacement in paediatric oncology patients with deep venous thrombosis: a multicentre study. Thromb. Haemost. 113, 434–435 (2015).
Journeycake, J. M. & Buchanan, G. R. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J. Clin. Oncol. 28, 4575–4580 (2006).
Pelland-Marcotte, M. C. et al. Thrombosis is associated with worse survival in children with acute lymphoblastic leukemia: a report from CYP-C. Am. J. Hematol. 96, 796–804 (2021).
Doiron, D., Raina, P. & Fortier, I. Linking Canadian population health data: maximizing the potential of cohort and administrative data. Can. J. Public Health 104, e258–e261 (2013).
Ulrich, E. H., So, G., Zappitelli, M. & Chanchlani, R. A review on the application and limitations of administrative health care data for the study of acute kidney injury epidemiology and outcomes in children. Front. Pediatr. 9, 742888 (2021).
Burles, K., Innes, G., Senior, K., Lang, E. & McRae, A. Limitations of pulmonary embolism ICD-10 codes in emergency department administrative data: let the buyer beware. BMC Med. Res. Methodol. 17, 89 (2017).
Alotaibi, G. S., Wu, C., Senthilselvan, A. & McMurtry, M. S. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc. Med. 20, 364–368 (2015).
Tamariz, L., Harkins, T. & Nair, V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol. Drug Saf. 21, 154–162 (2021).
Branchford, B. R., Gibson, E., Manco-Johnson, M. J. & Goldenberg, N. A. Sensitivity of discharge diagnosis ICD-9 codes for pediatric venous thromboembolism is greater than specificity, but still suboptimal for surveillance and clinical research. Thromb. Res. 129, 662–663 (2012).
https://www.pogo.ca/wp-content/uploads/2015/02/POGO_CC-Atlas-2-POGONIS_Feb-2015.pdf, accessed 7 April 2023.
https://www.ices.on.ca/Data-and-Privacy/ICES-data, accessed 7 April 2023.
Greenberg, M. L. et al. Childhood cancer registries in Ontario, Canada: lessons learned from a comparison of two registries. Int. J. Cancer 105, 88–91 (2003).
Pole, J. D. et al. Subsequent malignant neoplasms in pediatric cancer patients treated with and without hematopoietic SCT. Bone Marrow Transpl. 50, 721–726 (2015).
Pole, J. D., Gu, L. Y., Kirsh, V., Greenberg, M. L. & Nathan, P. C. Subsequent malignant neoplasms in a population-based cohort of pediatric cancer patients: a focus on the first 5 years. Cancer Epidemiol. Biomark. Prev. 24, 1585–1592 (2015).
Gupta, S. et al. Validity of administrative data in identifying cancer-related events in adolescents and young adults. Med. Care 56, e32–e38 (2018).
Athale, U. H. et al. Epidemiology and clinical risk factors predisposing to thromboembolism in children with cancer. Pediatr. Blood Cancer 51, 792–797 (2008).
Spavor, M. et al. Age at cancer diagnosis, non-O blood group and asparaginase therapy are independently associated with deep venous thrombosis in pediatric oncology patients: a risk model. Thromb. Res. 144, 27–31 (2016).
Halton, J. et al. Do children with central venous line dysfunction have increased risk of symptomatic thromboembolism compared to those without CVL-dysfunction, while on cancer therapy? Study protocol. BMC Cancer 12, 314 (2012).
Steliarova-Foucher, E., Stiller, C., Lacour, B. & Kaatsch, P. International classification of childhood cancer, third edition. Cancer 103, 1457–1467 (2005).
Walker, A. J. et al. Venous thromboembolism in children with cancer- a population-based cohort study. Thromb. Res. 133, 340–344 (2014).
Delate, T. et al. Outpatient use of low molecular weight heparin monotherapy for first-line treatment of venous thromboembolism in advanced cancer. Oncologist 17, 419–427 (2012).
Molnar, A. O. et al. Risk and complications of venous thromboembolism in dialysis patients. Nephrol. Dial. Transpl. 33, 874–880 (2018).
Bujang, M. A. & Adnan, T. H. Requirements for minimum sample size for sensitivity and specificity analysis. J. Clin. Diagn. Res. 10, YE01–YE06 (2016).
Fang, M. C. et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med. Care 55, e137–e143 (2017).
Quan, H. et al. IMECCHI investigators. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv. Res. 43, 1424–1441 (2008).
Monaghan, T. F. et al. Foundational statistical principles in medical research: sensitivity, specificity, positive predictive value, and negative predictive value. Medicine 57, 503 (2021).
Lucyk, K. et al. Administrative health data in Canada: lessons from history. BMC Med. Inf. Decis. Mak. 15, 69–74 (2015).
Acknowledgements
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This research was facilitated by the Pediatric Oncology Group of Ontario’s Networked Information System, financially supported by Ontario’s Ministry of Health and Long-Term Care. This study also received funding from Hamilton Health Sciences. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File. This research was facilitated by the Pediatric Oncology Group of Ontario’s Networked Information System, financially supported by Ontario’s Ministry of Health and Long-Term Care. Parts of this material are based on data and/or information compiled and provided by CIHI and the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Funding
This study was supported in part by Hamilton Health Sciences Foundation grant. The funder/sponsor did not participate in the work.
Author information
Authors and Affiliations
Contributions
Dr Uma Athale conceptualized and designed the study, collected data, drafted the initial manuscript, and critically reviewed and revised the manuscript. Dr Jacqueline Halton collected data, and critically reviewed and revised the manuscript. Anastasia Gayowsky carried out the analyses, and critically reviewed and revised the manuscript. Dr. Anthony Chan conceptualized the study, and critically reviewed and revised the manuscript. Dr Jason Pole conceptualized and designed the study, guided the analyses, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Consent statement
ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, with a consent waiver for health system evaluation and improvement. The study was approved by each of the institution’s research and ethics board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Athale, U., Halton, J., Gayowsky, A. et al. Development and validation of thromboembolism diagnostic algorithms in children with cancer from real-world data. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03082-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03082-x