Abstract
Background
We investigated the role of postnatal steroids on the severity of retinopathy of prematurity (ROP) and its impact on peripheral avascular retina (PAR).
Methods
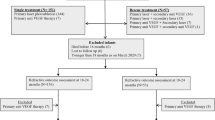
A retrospective cohort study of infants born at ≤32 weeks gestation and/or birth weight ≤1500 g. Demographics, the dose and duration of steroid treatment, and age when full retinal vascularization occurred were collected. The primary outcomes were the severity of ROP and time to full vascularization of the retina.
Results
A total of 1695 patients were enrolled, 67% of whom received steroid therapy. Their birth weight was 1142 ± 396 g and gestational age was 28.6 ± 2.7 weeks. The total hydrocortisone-equivalent dose prescribed was 28.5 ± 74.3 mg/kg. The total days of steroid treatment were 8.9 ± 35.1 days. After correction for major demographic differences, infants who received a higher cumulative dose of steroids for a longer duration had a significantly increased incidence of severe ROP and PAR (P < 0.001). For each day of steroid treatment, there was a 3.2% increase in the hazard of the severe form of ROP (95% CI: 1.022–1.043) along with 5.7% delay in achieving full retinal vascularization (95% CI: 1.04–1.08) (P < 0.001).
Conclusion
Cumulative dose and duration of postnatal steroid use were independently associated with the severity of ROP and PAR. Thus, postnatal steroids should be used very prudently.
Impact
-
We report ROP outcomes in a large cohort of infants from two major healthcare systems where we have studied the impact of postnatal steroids on the severity of ROP, growth, and development of retinal vessels.
-
After correcting our data for three major outcome measures, we show that high-dose postnatal steroids used for a prolonged duration of time are independently associated with severe ROP and delay in retinal vascularization.
-
Postnatal steroids impact the visual outcomes of VLBW infants significantly, so their clinical use needs to be moderated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors agree to share deidentified patient data and statistical analysis on request by other investigators after obtaining permission from each healthcare system. Data will be made available by the corresponding author.
References
Magro Malosso, E. R., Saccone, G., Simonetti, B., Squillante, M. & Berghella, V. US trends in abortion and preterm birth. J. Matern Fetal Neonatal Med. 31, 2463–2467 (2018).
Bell, E. F. et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA 327, 248–263 (2022).
Ferre, C., Callaghan, W., Olson, C., Sharma, A. & Barfield, W. Effects of maternal age and age-specific preterm birth rates on overall preterm birth rates – United States, 2007 and 2014. MMWR Morb. Mortal. Wkly Rep. 65, 1181–1184 (2016).
Chiang, M. F. et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 128, e51–e68 (2021).
Fernandez, E. F. & Watterberg, K. L. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 29, S44–S49 (2009).
Quintos, J. B. & Boney, C. M. Transient adrenal insufficiency in the premature newborn. Curr. Opin. Endocrinol. Diabetes Obes. 17, 8 (2010).
Ng, P. C. et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch. Dis. Child Fetal Neonatal Ed. 89, F119–F126 (2004).
Ng, P. C. et al. Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch. Dis. Child Fetal Neonatal Ed. 84, F122–F124 (2001).
Watterberg, K. L., Gerdes, J. S. & Cook, K. L. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr. Res. 50, 190–195 (2001).
Watterberg, K. L. et al. Adrenal function links to early postnatal growth and blood pressure at age 6 in children born extremely preterm. Pediatr. Res. 86, 339–347 (2019).
Watterberg, K. L., Scott, S. M., Backstrom, C., Gifford, K. L. & Cook, K. L. Links between early adrenal function and respiratory outcome in preterm infants: airway inflammation and patent ductus arteriosus. Pediatrics 105, 320–324 (2000).
Doyle, L. W., Ehrenkranz, R. A. & Halliday, H. L. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst. Rev. CD001145 (2014).
Doyle, L. W., Ehrenkranz, R. A. & Halliday, H. L. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst. Rev. CD001146 (2014).
Arad, I. et al. Long-term cognitive benefits of antenatal corticosteroids for prematurely born children with cranial ultrasound abnormalities. Am. J. Obstet. Gynecol. 186, 818–825 (2002).
Doyle, L. W., Halliday, H. L., Ehrenkranz, R. A., Davis, P. G. & Sinclair, J. C. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J. Pediatr. 165, 1258–1260 (2014).
Committee on Obstetric Practice. Committee Opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130, e102–e109 (2017).
Aly, H., Othman, H. F., Munster, C., Das, A. & Sears, J. The US national trend for retinopathy of prematurity. Am. J. Perinatol. 14, 1569–1576 (2022).
Kim, S. J. et al. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv. Ophthalmol. 63, 618–637 (2018).
Mirabelli, P. et al. Genome-wide expression differences in anti-VEGF and dexamethasone treatment of inflammatory angiogenesis in the rat cornea. Sci. Rep. 7, 7616 (2017).
Smith, L. E. Through the eyes of a child: understanding retinopathy through ROP the friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 49, 5177–5182 (2008).
Bonafiglia, E. et al. Early and late onset sepsis and retinopathy of prematurity in a cohort of preterm infants. Sci. Rep. 12, 11675 (2022).
Chang, J. W. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS One 14, e0219934 (2019).
Diggikar, S., Aradhya, A. S., Swamy, R. S., Namachivayam, A. & Chandrasekaran, M. Effect of enteral long-chain polyunsaturated fatty acids on retinopathy of prematurity: a systematic review and meta-analysis. Neonatology 119, 547–557 (2022).
Das, A. et al. Effect of fluctuation of oxygenation and time spent in the target range on retinopathy of prematurity in extremely low birth weight infants. J. Neonatal Perinat. Med. 11, 257–263 (2018).
Cuculich, P. S., DeLozier, K. A., Mellen, B. G. & Shenai, J. P. Postnatal dexamethasone treatment and retinopathy of prematurity in very-low-birth-weight neonates. Biol. Neonate 79, 9–14 (2001).
Haroon Parupia, M. F. & Dhanireddy, R. Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopathy of prematurity and progression to laser therapy in extremely low-birth-weight infants. J. Perinatol. 21, 242–247 (2001).
Movsas, T. Z., Spitzer, A. R. & Gewolb, I. H. Postnatal corticosteroids and risk of retinopathy of prematurity. J. AAPOS 20, 348–352 (2016).
Öhnell, H. M., Andreasson, S. & Gränse, L. Dexamethasone eye drops for the treatment of retinopathy of prematurity. Ophthalmol. Retina 6, 181–182 (2022).
Tao, K. Postnatal administration of systemic steroids increases severity of retinopathy in premature infants. Pediatr. Neonatol. 63, 220–226 (2022).
Sun, Y., Hellstrom, A. & Smith, L. E. H. in Fanaroff & Martin’s Neonatal-Perinatal Medicine Vol. 2 (eds Martin, R.J., Fanaroff, A.A. & Walsh, M.C.) Ch. 1767–1774 (Elsevier, Saunders, 2015).
Ozgur Gursoy, O., Gurer, H. G., Yildiz Eren, C., Erdogan Ozgur, P. & Gursoy, H. The association of various obstetric and perinatal factors with retinopathy of prematurity. Int. Ophthalmol. 42, 2719–2728 (2022).
Hartnett, M. E. Discovering mechanisms in the changing and diverse pathology of retinopathy of prematurity: The Weisenfeld Award Lecture. Invest. Ophthalmol. Vis. Sci. 60, 1286–1297 (2019).
Quinlivan, J. A., Beazley, L. D., Evans, S. F., Newnham, J. P. & Dunlop, S. A. Retinal maturation is delayed by repeated, but not single, maternal injections of betamethasone in sheep. Eye (Lond.) 14, 93–98 (2000).
Spandau, U. H., Sauder, G., Schubert, U., Hammes, H. P. & Jonas, J. B. Effect of triamcinolone acetonide on proliferation of retinal endothelial cells in vitro and in vivo. Br. J. Ophthalmol. 89, 745–747 (2005).
Spandau, U. H., Vom Hagen, F., Hammes, H. P. & Jonas, J. B. Effect of intravitreal triamcinolone acetonide on retinal apoptosis in experimental retinal neovascularization. Graefes Arch. Clin. Exp. Ophthalmol. 246, 1069–1070 (2008).
Hartnett, M. E. et al. Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Invest. Ophthalmol. Vis. Sci. 47, 4975–4982 (2006).
Malaeb, S. N. & Stonestreet, B. S. Steroids and injury to the developing brain: net harm or net benefit? Clin. Perinatol. 41, 191–208 (2014).
Zeng, L. et al. Corticosteroids for the prevention of bronchopulmonary dysplasia in preterm infants: a network meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 103, F506–F511 (2018).
Baisden, B., Sonne, S., Joshi, R. M., Ganapathy, V. & Shekhawat, P. S. Antenatal dexamethasone treatment leads to changes in gene expression in a murine late placenta. Placenta 28, 1082–1090 (2007).
Newell-Price, J. D. C. & Auchus, R. J. The Adrenal Cortx. In Williams Textbook of Endocrinology (ed. Melmed, S. & Auchus R. J. et al.) Ch. 15, 480–541 (Elsevier, 2020).
Nakanishi, H. et al. Trends in the neurodevelopmental outcomes among preterm infants from 2003–2012: a retrospective cohort study in Japan. J. Perinatol. 38, 917–928 (2018).
Aly, H., Othman, H. F., Munster, C., Das, A. & Sears, J. The U.S. national trend for retinopathy of prematurity. Am. J. Perinatol. 29, 1569–1576 (2022).
Hwang, J. H., Lee, E. H. & Kim, E. A. Retinopathy of prematurity among very-low-birth-weight infants in Korea: incidence, treatment, and risk factors. J. Korean Med. Sci. 30, S88–S94 (2015).
Siffel, C., Hirst, A. K., Sarda, S. P., Kuzniewicz, M. W. & Li, D. K. The clinical burden of extremely preterm birth in a large medical records database in the United States: mortality and survival associated with selected complications. Early Hum. Dev. 171, 105613 (2022).
Hsu, H. T. et al. Late vitreoretinal complications of regressed retinopathy of prematurity: retinal break, vitreous hemorrhage and retinal detachment. Ophthalmol. Retina 7, 72–80 (2022).
Smolkin, T. et al. Late postnatal systemic steroids predispose to retinopathy of prematurity in very-low-birth-weight infants: a comparative study. Acta Paediatr. 97, 322–326 (2008).
Petursdottir, D., Holmstrom, G. & Larsson, E. Visual function is reduced in young adults formerly born prematurely: a population-based study. Br. J. Ophthalmol. 104, 541–546 (2020).
Kistner, A., Jacobson, L., Ostergren, J. & Hellstrom, A. Retinopathy of prematurity is associated with increased systolic blood pressure in adults who were born preterm. Neonatology 112, 87–91 (2017).
Holmstrom, G. et al. New modifications of Swedish ROP guidelines based on 10-year data from the Swedrop Register. Br. J. Ophthalmol. 104, 943–949 (2020).
Fung, A. T. et al. Local delivery of corticosteroids in clinical ophthalmology: a review. Clin. Exp. Ophthalmol. 48, 366–401 (2020).
Chung, I. Y. et al. Protective effects of triamcinolone acetonide upon the upregulation and phosphorylation of GAP 43 in an animal model of retinopathy of prematurity. Acta Ophthalmol. 88, e217–e221 (2010).
Watterberg, K. L. American Academy of Pediatrics. Committee on Fetus and Newborn Policy statement-postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 126, 800–808 (2010).
Cummings, J. J. P. & Aap, A. K. Committee on Fetus and Newborn Postnatal corticosteroids to prevent or treat chronic lung disease following preterm birth. Pediatrics 149, e2022057530 (2022).
Shukla, A. et al. Comparison of biphasic vs static oxygen saturation targets among infants with retinopathy of prematurity. JAMA Ophthalmol. 137, 417–423 (2019).
Lawas-Alejo, P. A., Slivka, S., Hernandez, H., Bry, K. & Hallman, M. Hyperoxia and glucocorticoid modify retinal vessel growth and interleukin-1 receptor antagonist in newborn rabbits. Pediatr. Res. 45, 313–317 (1999).
Sears, J. E. & Hoppe, G. Triamcinolone acetonide destabilizes VEGF mRNA in muller cells under continuous cobalt stimulation. Invest Ophthalmol. Vis. Sci. 46, 4336–4341 (2005).
Funding
The study was funded in part by NIH grant UL1 TR002548 from NCATS/NIH, which provided us REDcap (Research electronic data capture) software used for data storage, analysis, and sharing.
Author information
Authors and Affiliations
Contributions
Data collection was performed by the following authors: M.A.M.A., N.K., H.K., C.M., M.P., and M.A.A.F. All authors participated in the concept and design, analysis and interpretation of data, and drafting and revising of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This is a retrospective cohort study so both IRBs exempted us from obtaining consent from the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shekhawat, P.S., Ali, M.A.M., Kannekanti, N. et al. Impact of postnatal steroids on peripheral avascular retina and severity of retinopathy of prematurity. Pediatr Res 94, 1966–1972 (2023). https://doi.org/10.1038/s41390-023-02673-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02673-4