Abstract
Background
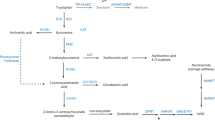
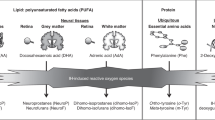
Hypoxemia is a physiological manifestation of immature respiratory control in preterm neonates, which is likely impacted by neurotransmitter imbalances. We investigated relationships between plasma levels of the neurotransmitter serotonin (5-HT), metabolites of tryptophan (TRP), and parameters of hypoxemia in preterm neonates.
Methods
TRP, 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), and kynurenic acid (KA) were analyzed in platelet-poor plasma at ~1 week and ~1 month of life from a prospective cohort of 168 preterm neonates <31 weeks gestational age (GA). Frequency of intermittent hypoxemia (IH) events and percent time hypoxemic (<80%) were analyzed in a 6 h window after the blood draw.
Results
At 1 week, infants with detectable plasma 5-HT had fewer IH events (OR (95% CI) = 0.52 (0.29, 0.31)) and less percent time <80% (OR (95% CI) = 0.54 (0.31, 0.95)) compared to infants with undetectable 5-HT. A similar relationship occurred at 1 month. At 1 week, infants with higher KA showed greater percent time <80% (OR (95% CI) = 1.90 (1.03, 3.50)). TRP, 5-HIAA or KA were not associated with IH frequency at either postnatal age. IH frequency and percent time <80% were positively associated with GA < 29 weeks.
Conclusions
Circulating neuromodulators 5-HT and KA might represent biomarkers of immature respiratory control contributing to hypoxemia in preterm neonates.
Impact
-
Hypoxemia events are frequent in preterm infants and are associated with poor outcomes.
-
Mechanisms driving hypoxemia such as immature respiratory control may include central and peripheral imbalances in modulatory neurotransmitters.
-
This study found associations between the plasma neuromodulators serotonin and kynurenic acid and parameters of hypoxemia in preterm neonates.
-
Imbalances in plasma biomarkers affecting respiratory control may help identify neonates at risk of short- and long-term adverse outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Di Fiore, J. M., MacFarlane, P. M. & Martin, R. J. Intermittent hypoxemia in preterm infants. Clin. Perinatol. 46, 553–565 (2019).
Di Fiore, J. M. & Raffay, T. M. The relationship between intermittent hypoxemia events and neural outcomes in neonates. Exp. Neurol. 342, 113753 (2021).
Jensen, E. A. et al. Association between intermittent hypoxemia and severe bronchopulmonary dysplasia in preterm infants. Am. J. Respir. Crit. Care Med. 204, 1192–1199 (2021).
Poets, C. F. et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 314, 595–603 (2015).
Ferrante, G., Carota, G., Li Volti, G. & Giuffrè, M. Biomarkers of oxidative stress for neonatal lung disease. Front. Pediatr. 9, 618867 (2021).
Sánchez-Illana, Á. et al. Oxidative stress biomarkers in the preterm infant. Adv. Clin. Chem. 102, 127–189 (2021).
Sánchez-Illana, Á. et al. Novel free-radical mediated lipid peroxidation biomarkers in newborn plasma. Anal. Chim. Acta 996, 88–97 (2017).
Muñoz-Ortiz, J., Muñoz-Ortiz, E., López-Meraz, M. L., Beltran-Parrazal, L. & Morgado-Valle, C. Pre-Bötzinger complex: Generation and modulation of respiratory rhythm. Neurologia 34, 461–468 (2019).
Ramirez, J. M. et al. The cellular building blocks of breathing. Compr. Physiol. 2, 2683–2731 (2012).
Nurse, C. A. & Piskuric, N. A. Signal processing at mammalian carotid body chemoreceptors. Semin. Cell Dev. Biol. 24, 22–30 (2013).
Prabhakar, N. R. & Peng, Y. J. Oxygen sensing by the carotid body: past and present. Adv. Exp. Med. Biol. 977, 3–8 (2017).
Teran, F. A., Massey, C. A. & Richerson, G. B. Serotonin neurons and central respiratory chemoreception: where are we now? Prog. Brain Res. 209, 207–233 (2014).
Bravo, K., Eugenín, J. & Llona, I. Neurodevelopmental effects of serotonin on the brainstem respiratory network. Adv. Exp. Med. Biol. 1015, 193–216 (2017).
Cummings, K. J. & Hodges, M. R. The serotonergic system and the control of breathing during development. Respir. Physiol. Neurobiol. 270, 103255 (2019).
Modoux, M., Rolhion, N., Mani, S. & Sokol, H. Tryptophan metabolism as a pharmacological target. Trends Pharm. Sci. 42, 60–73 (2021).
Kinney, H. C. & Haynes, R. L. The serotonin brainstem hypothesis for the sudden infant death syndrome. J. Neuropathol. Exp. Neurol. 78, 765–779 (2019).
Malloy, M. H. & Hoffman, H. J. Prematurity, sudden infant death syndrome, and age of death. Pediatrics 96, 464–471 (1995).
Malloy, M. H. Sudden infant death syndrome among extremely preterm infants: United States 1997-1999. J. Perinatol. 24, 181–187 (2004).
Young, J. O., Geurts, A., Hodges, M. R. & Cummings, K. J. Active sleep unmasks apnea and delayed arousal in infant rat pups lacking central serotonin. J. Appl. Physiol. 123, 825–834 (2017).
Hodges, M. R., Wehner, M., Aungst, J., Smith, J. C. & Richerson, G. B. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci. 29, 10341–10349 (2009).
Hodges, M. R. & Richerson, G. B. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J. Appl. Physiol. 108, 1425–1432 (2010).
MacFarlane, P. M., Ribeiro, A. P. & Martin, R. J. Carotid chemoreceptor development and neonatal apnea. Respir. Physiol. Neurobiol. 185, 170–176 (2013).
Badawy, A. A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 112, 248–263 (2017).
Abrahams, T. P., Taveira DaSilva, A. M., Hamosh, P., McManigle, J. E. & Gillis, R. A. Cardiorespiratory effects produced by blockade of excitatory amino acid receptors in cats. Eur. J. Pharmacol. 238, 223–233 (1993).
Guyenet, P. G., Mulkey, D. K., Stornetta, R. L. & Bayliss, D. A. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci. 25, 8938–8947 (2005).
Moreira, T. S., Takakura, A. C., Colombari, E. & Guyenet, P. G. Central chemoreceptors and sympathetic vasomotor outflow. J. Physiol. 577, 369–386 (2006).
Mutolo, D., Bongianni, F., Nardone, F. & Pantaleo, T. Respiratory responses evoked by blockades of ionotropic glutamate receptors within the Bötzinger complex and the pre-Bötzinger complex of the rabbit. Eur. J. Neurosci. 21, 122–134 (2005).
Silva, N. T., Nalivaiko, E., da Silva, L. G. & Haibara, A. S. Excitatory amino acid receptors in the dorsomedial hypothalamic area contribute to the chemoreflex tachypneic response. Respir. Physiol. Neurobiol. 212–214, 1–8 (2015).
Tolentino-Silva, F. P., Russo, A. K., Cravo, S. L. & Lopes, O. U. Respiratory effects of kynurenic acid microinjected into the ventromedullary surface of the rat. Braz. J. Med. Biol. Res. 31, 1339–1343 (1998).
Schumacher, R. E., Farrell, P. M. & Olson, E. B. Jr Circulating 5-hydroxytryptamine concentrations in preterm newborns. Pediatr. Pulmonol. 3, 117–122 (1987).
Di Fiore, J. M. et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 157, 69–73 (2010).
Martin, R. J., Di Fiore, J. M., Macfarlane, P. M. & Wilson, C. G. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv. Exp. Med. Biol. 758, 351–358 (2012).
Dennery, P. A. et al. Pre-Vent: the prematurity-related ventilatory control study. Pediatr. Res. 85, 769–776 (2019).
Flynn, J. T. & Bancalari, E. On “supplemental therapeutic oxygen for prethreshold retinopathy of prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes”. J. AAPOS 4, 65–66 (2000).
Lario, S. et al. Plasma sample based analysis of gastric cancer progression using targeted metabolomics. Sci. Rep. 7, 17774 (2017).
Gardiner, J. C., Luo, Z. & Roman, L. A. Fixed effects, random effects and GEE: what are the differences? Stat. Med. 28, 221–239 (2009).
Heagerty, P. J. Marginally specified logistic-normal models for longitudinal binary data. Biometrics 55, 688–698 (1999).
McCullagh, P. Regression models for ordinal data. J. R. Stat. Soc. Ser. B (Methodol.) 42, 109–127 (1980).
Clarke, G., Stone, T. W. & Schwarcz, R. The kynurenine pathway: towards metabolic equilibrium. Neuropharmacology 112, 235–236 (2017).
Schwarcz, R. & Stone, T. W. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112, 237–247 (2017).
O’Connor, J. C. et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 14, 511–522 (2009).
Lal, C. V. & Ambalavanan, N. Biomarkers, early diagnosis, and clinical predictors of bronchopulmonary dysplasia. Clin. Perinatol. 42, 739–754 (2015).
Sahni, M. et al. Novel biomarkers of bronchopulmonary dysplasia and bronchopulmonary dysplasia-associated pulmonary hypertension. J. Perinatol. 40, 1634–1643 (2020).
Bhandari, A. & Bhandari, V. Biomarkers in bronchopulmonary dysplasia. Paediatr. Respir. Rev. 14, 173–179 (2013).
Murugesan, A. et al. Serum serotonin levels in patients with epileptic seizures. Epilepsia 59, e91–e97 (2018).
Haynes, R. L. et al. High serum serotonin in sudden infant death syndrome. Proc. Natl Acad. Sci. USA 114, 7695–7700 (2017).
Sibbald, W., Peters, S. & Lindsay, R. M. Serotonin and pulmonary hypertension in human septic ARDS. Crit. Care Med. 8, 490–494 (1980).
Nurse, C. A. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp. Physiol. 95, 657–667 (2010).
Doi, A. & Ramirez, J. M. Neuromodulation and the orchestration of the respiratory rhythm. Respir. Physiol. Neurobiol. 164, 96–104 (2008).
Hodges, M. R. & Richerson, G. B. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir. Physiol. Neurobiol. 164, 222–232 (2008).
Schwarcz, R., Bruno, J. P., Muchowski, P. J. & Wu, H. Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 (2012).
Moraes, D. J., Zoccal, D. B. & Machado, B. H. Sympathoexcitation during chemoreflex active expiration is mediated by L-glutamate in the RVLM/Bötzinger complex of rats. J. Neurophysiol. 108, 610–623 (2012).
Solomon, I. C. Ionotropic excitatory amino acid receptors in pre-Botzinger complex play a modulatory role in hypoxia-induced gasping in vivo. J. Appl. Physiol. 96, 1643–1650 (2004).
Liu, G., Feldman, J. L. & Smith, J. C. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J. Neurophysiol. 64, 423–436 (1990).
Fairchild, K. D., Nagraj, V. P., Sullivan, B. A., Moorman, J. R. & Lake, D. E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 85, 987–993 (2019).
Raffay, T. M. et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr. Res. 85, 318–323 (2019).
Bonnin, A. & Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 197, 1–7 (2011).
Dobson, N. R. et al. Caffeine decreases intermittent hypoxia in preterm infants nearing term-equivalent age. J. Perinatol. 37, 1135–1140 (2017).
Egri, C., Dunbar, M. & Horvath, G. A. Correlation between salivary, platelet and central serotonin levels in children. Can. J. Neurol. Sci. 47, 214–218 (2020).
Audhya, T., Adams, J. B. & Johansen, L. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim. Biophys. Acta 1820, 1496–1501 (2012).
Acknowledgements
The authors wish to thank the participating families, the neonatal research staff, specifically Advanced Clinical Research Nurse Ms. Arlene Zadell, and our scientific collaborators at Institut des Biomolecules Max Mousseron, UMR 5247 CNRS, ENSCM, Universite de Montpellier, Montpellier, France, specifically Camille Oger, Jean-Marie Galano, and Thierry Durand.
Funding
This study was supported by grants from the National Institutes of Health [U01HL133643, U01HL133708 and UL1TR002548. A.S.I. acknowledges the support of RETICS from the Health Research Institute Carlos III, Spain (ISCIII) - European Regional Development Fund (FEDER) [RD16/0022/001], the PFIS grant from ISCIII (Ministry of Science and Innovation) [FI16/00380], and Margarita Salas grant [UP2021-044-MS21-084] from the Ministry of Universities of the Government of Spain, financed by the European Union, NextGeneration EU. M.V. acknowledges the support of RETICS [PN 2018-2021 (Spain)], ISCIII, Spain - Sub-Directorate General for Research Assessment and Promotion and FEDER [RD16/0022], and ISCIII (Ministry of Science and Innovation) [PI20/00964]. J.K. acknowledges the support of ISCIII, Spain and co-funded by the European Union [CPII21/00003].
Author information
Authors and Affiliations
Contributions
P.M.M., A.M.H., T.M.R., J.M.D., M.V., and R.J.M. made substantial contributions to conception and study design, acquisition of data and analysis, and interpretation of data. G.Q., A.S.I., J.K., J.D.P.-R., C.T., Z.C., and N.M. made substantial contributions to data acquisition and analysis. All authors contributed to revising the article and gave critically important intellectual input. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written parental consent was obtained. Oversight was provided by a local institutional review board and observational and safety monitoring board, appointed by the NHLBI.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
MacFarlane, P.M., Martin, R.J., Di Fiore, J.M. et al. Plasma serotonergic biomarkers are associated with hypoxemia events in preterm neonates. Pediatr Res 94, 1436–1443 (2023). https://doi.org/10.1038/s41390-023-02620-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02620-3
This article is cited by
-
Senior investigator biocommentary: Max Vento
Pediatric Research (2024)