Abstract
Background
Inadequate sleep duration has been suggested as a chronic stressor associated with changes in telomere length (TL). This study aimed to explore the association between sleep duration and TL using the INMA birth cohort study data.
Methods
A total of 1014 children were included in this study (cross-sectional: 686; longitudinal: 872). Sleep duration (h/day) was reported by caregivers at 4 years and classified into tertiles (7–10 h/day; >10–11 h/day; >11–14 h/day). Leucocyte TL at 4 and 7–9 years were measured using quantitative PCR methods. Multiple robust linear regression models, through log-level regression models, were used to report the % of difference among tertiles of sleep duration.
Results
In comparison to children who slept between >10 and 11 h/day, those in the highest category (more than 11 h/day) had 8.5% (95% CI: 3.56–13.6) longer telomeres at 4 years. Longitudinal analysis showed no significant association between sleep duration at 4 years and TL at 7–9 years.
Conclusion
Children who slept more hours per day had longer TL at 4 years independently of a wide range of confounder factors. Environmental conditions, such as sleep duration, might have a major impact on TL during the first years of life.
Impact
-
Telomere length was longer in children with longer sleep duration (>11 h/day) independently of a wide range of confounder factors at age 4 and remained consistent by sex.
-
Sleep routines are encouraged to promote positive child development, like the number of hours of sleep duration.
-
Considering the complex biology of telomere length, future studies still need to elucidate which biological pathways might explain the association between sleep duration and telomere length.
Similar content being viewed by others
Introduction
Sleep is a necessary physiological process and has a critical role in promoting balanced health.1 In children, adequate sleep is associated with normal growth, wellbeing and different development domains such as nutrition, hygiene, communication and physical contact.2,3 Inadequate sleep, instead—defined mainly as the number of hours a child sleeps—negatively impacts cognitive functions, socioemotional domains, early childhood development and physical health.2 The American Academy of Sleep Medicine recommends sleeping 10–13 h per day for children between 3 and 5 years old to reach their full developmental potential.3 However, not all children meet this recommendation. For instance, 34.9% of American children and adolescents aged 4 months to 17 years reported sleeping less than the recommendations for their age.4 In Spain, Ruiter et al. estimated that sleep duration in children between 2 and 14 years had decreased by 20 min in the last decades and that only 55% of children were sleeping enough hours per day.5
In addition to the aforementioned consequences of sleep disturbance, inadequate sleep duration has been suggested as a chronic stressor associated with changes in telomere length.6,7,8 Telomeres are nucleoprotein structures containing repeat sequences of tandem TTAGGG DNA stretches that protect chromosome ends from illicit DNA repair. Naturally, they shorten over time; however, they are susceptible to faster shortening under stressors. Previous studies have identified that shorter telomeres are associated with a higher risk of adverse health outcomes and have been identified as a useful ageing biomarker.9
Although studies evaluating telomere length in children are limited, previous works highlighted the association between childhood abuse, early life adversity, childhood socioeconomic status and maternal factors (such as depression, smoking and inheritance) with telomere length.10,11 Regarding sleep, studies conducted in adults have shown that poor sleep quality is associated with shorter telomere length.6,7,8 In children, two studies evaluated the potential association between sleep duration and telomere length. However, results from both studies are inconclusive since one evidenced a positive association and the other no association between these two variables.12,13 Considering that the literature has proposed that the environmental conditions during adulthood might have less impact over telomere length than those during childhood14,15—and the poorly investigated role of sleep in children—this study aimed to explore the association between sleep duration and telomere length using data from the INMA birth cohort study.
Methods
This study was carried out using data from the INMA birth cohort study (Environment and Childhood; in Spanish: INfancia y Medio Ambiente;). The INMA project’s main aim is to investigate the role of environmental factors during pregnancy and early life and their effects on child growth and development. More details about the INMA project can be found online https://www.proyectoinma.org/ and have been published elsewhere.16
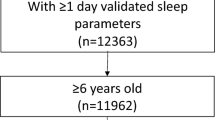
In brief, pregnant women from the general population were recruited between 2004 and 2008 in four areas of Spain (Asturias, Gipuzkoa, Sabadell and Valencia) using the following inclusion criteria: ≥16 years old, singleton pregnancy, no assisted conception, intention to deliver at the reference hospital and to have no communication problems. Of the original sample, 1014 children had available data on the exposure (sleep at 4 years), at least one of the outcomes (telomere length at 4 years, or at 7–9 years; ‘hereafter: 7–9’) and covariates and were, therefore, included in the analyses (Fig. 1). Of them, 686 and 875 children had information for telomere length at 4 and 7–9 years, respectively (Fig. 1).
Sleep categories—exposure
Caregivers (parents/legal tutors) reported the child’s sleep time (h/day) in the assessment carried out at 4 years using questionnaires. During the evaluation, the examiner asked, ‘how many hours does your child sleep during the week (h/day)?’ and ‘how many hours does your child sleep during the weekend (h/day)?’ The average sleep per day was estimated as the sum of hours during the week and weekend divided by seven using these two questions as follows: (((weekday sleep time ×5)+(weekend sleep time ×2))/7). The American Academy of Sleep Medicine recommends children sleep between 10 and 13 h/day at 4 years.3 However, only 7 children were reported as sleeping more than 13 h/day, whilst 89 less than 10 h/day, representing a relatively homogeneous sample. Consequently, the average per day was categorised in tertiles following the participants’ distribution as follows: (i) 7–10 h/day, (ii) 10.02–11 h/day (hereafter, ‘>10–11 h/day’) and (iii) 11.03–14 h/day (hereafter, ‘>11–14 h/day’).
Leucocyte telomere length—outcome
Leucocyte telomere length (LTL) at 4 years was available in Gipuzkoa, Sabadell and Asturias (average age: 4.4 years, standard deviation [SD] 0.2 years; interquartile range 4.4–4.5 years). The cross-sectional analysis was restricted to these participants only (Fig. 1). On the other hand, Gipuzkoa, Asturias and Valencia had available telomere data at 7 years, while Sabadell at 9 years only (average age: 8.2 years; SD: 0.6 years; interquartile range: 7.7–9.2 years). Therefore, these outcomes were pooled together to create the variable LTL at 7–9 years. The longitudinal analysis was restricted to these participants only (Fig. 1).
Blood samples were collected during clinical examination and properly stored in EDTA tubes. At 4 years, DNA was extracted from blood using the Flexigen AGKT-WB-640 (Qiagen) kit in Gipuzkoa samples, Chemagen kit (Perkin Elmer) in Sabadell and from buffy coat applying the QIAamp DNA Mini Kit (Qiagen) in Asturias. At 7–9 years, DNA was extracted from buffy coats using the aforementioned kits for Gipuzkoa, Sabadell and Asturias. In Valencia, DNA was extracted from buffy coats using the Chemagen kit (Perkin Elmer).17
As described in Supplementary methods, LTL was determined using quantitative PCR methods. Different single-copy gene primers were used to assess LTL at 9 years in the Sabadell cohort samples.18 Relative LTL was determined separately for each cohort and normalised separately using qBase software (Biogazelle, Zwijnaarde, Belgium) and expressed as the ratio of telomere copy number to single-copy gene number (T/S) relative to the average T/S ratio of the cohort sample set. The reliability of the applied protocol was assessed by interclass correlation coefficients of triplicate measures (T/S ratios, telomere copy number and single-copy gene number measures).
Covariates
Age (calculated from the date of birth and assessment at 4 years), sex (female or male), the cohort of origin (Gipuzkoa, Sabadell, Asturias or Valencia), blood extraction date (the day when telomere information was collected; then codified as the season of extraction), mother’s social class, parity (number of previous children, classified as 0 or ≥1), adherence to a relative Mediterranean Diet Score (rMED) and television (TV) time (reported by caregivers regarding the total hours during the week and weekend watching TV/videos, i.e., screen time) were the covariates included in the main analyses. rMED was previously published in children19 and is based on the Buckland et al. index excluding alcohol consumption20 since this study was restricted to children. The dietary index was calculated using the food intake of a validated food frequency questionnaire of eight components vegetables (excluding potatoes), fruit (including nuts, seeds and fruit juices), legumes, cereals (including whole grains and bread), fish (including seafood), meat (including processed meat), dairy products (including low-fat and high-fat products) and olive oil. Each rMED component was calculated in grams per 1000 kcal/day and divided into tertiles of intake.
Statistical analyses
Descriptive characteristics by children’s sleep categories are presented as median with their respectively interquartile range for quantitative variables. For categorical variables, data are reported as frequencies with their respective percentages. The distribution of the continuous variables was checked using the Lilliefors correction of the Kolmogorov–Smirnov test and compared using ANOVA or Kruskal–Wallis and χ2 tests, both for main covariates and additional descriptive variables used in the sensitivity analyses.
Associations were initially analysed using meta-analytic techniques to obtain combined estimates to quantify the heterogeneity among the study cohorts. The heterogeneity was quantified using I2 statistics in R.21 Since all I2 values obtained for the primary outcomes were <50%, we analysed adding the cohort variable to the adjustment of all the models (data not shown).
Associations between sleep categories and LTL at 4 years were investigated using multiple robust linear regression models, through log-level regression models, where the LTL was log10-transformed. Therefore, the results are reported as % difference and their respectively 95% CI. Children whose caregivers reported sleeping between >10 and 11 h/day were used as the reference group. Same analyses were performed when LTL at 7–9 years was used as the outcome of interest.
All analyses were adjusted using three incremental models: Model 1, adjusted for blood date (at 4 or 7–9 years, according to the outcome of interest), cohort, age at sleep assessment and sex of the child. Model 2: as per model 1, but also for social class and parity of the mother at baseline assessment. Model 3: as per model 2, but also for the rMED and TV time. These potential confounder factors were selected based on previous literature and also in those variables with p values <0.20 in the individual bivariate analyses at 4 and 7–9 years and those that changed the magnitude of the main effect magnitude by 10% using a backward-forward elimination procedure (data not shown).22
Finally, to investigate whether the association differed by sex, the analyses were repeated and stratified by sex (male and female) using the maximally adjusted model.
R 4.0.5 (packages ‘robustbase’, ‘nortest’, ‘meta’, ‘lmtest’, ‘foreign’, ‘car’, ‘gdata’) and Stata 17 were used to perform the statistical analyses. A p value lower than 0.05 was considered statistically significant.
Results
Cohort characteristics by sleep categories are presented in Table 1. A total of 489 (48.2%) caregivers reported their children slept between 7 and 10 h/day, while only 132 (13.0%) children were reported sleeping more than 11 h per day. Overall, and compared to those in the lowest sleep category (7–10 h/day), children who slept more than 11 h per day were more likely to be male and from Asturias and their mothers were more likely to belong to a lower social class (IV+V). They also tended to have a better rMED and watch fewer TV hours per week. More information regarding the children’s characteristics is available in Table 1.
Associations between sleep categories and LTL measured as a percentage difference at 4 and 7–9 years are presented in Table 2. Compared to those in the medium category (sleep between >10 and 11 h/day), children in the highest category (more than 11 h/day) had 6.9% (95% CI: 1.94–12.1) longer telomeres at 4 years (model 1). After further adjusting the model for other sociodemographic and lifestyle factors (models 2 and 3), the percentage difference at 4 years was even higher in this group (% difference: 8.48, 95% CI: 3.56–13.6). On the other hand, children in the lowest sleep category showed 2.2% longer LTL compared to their counterparts (model 3); however, this association was non-significant (p = 0.162). Regarding sleep duration at 4 years and LTL at 7–9 years, both children in the lowest and highest category showed longer LTL when compared to the models that evaluated LTL at the age of 4 years (Table 2), although the estimates were not statistically significant.
Finally, when the association was stratified by sex, similar patterns of associations were identified for sleep and telomere at 4 years (Supplementary Table 1). In the cross-sectional analyses, analyses remained significant for both sexes. Yet, boys had longer telomeres at 4 years than their counterparts (% differenceboys: 10, 95% CI: 2.97–17.8 and % differencegirls: 7.03, 95% CI: 0.24–14.3). No differences were identified in the longitudinal analysis by sex (Supplementary Table 1).
Discussion
This study showed that, compared with children whose caregivers reported they slept between >10 and 11 h/day, LTL was longer in those in the highest sleep category (>11 h/day) independently of a wide range of confounder factors at 4 years of age. This finding remained consistent by sex. Notwithstanding the above, we did not observe a significant association between sleep duration at 4 years and LTL later in childhood (7–9 years). Other unmeasured confounder factors could also explain the lack of significant association at this age. Yet, as previous authors have proposed, environmental conditions might have a major impact on telomere length during the first years of life.14
During the first 4 years of life, there is a rapid decline in LTL because of proliferative cells’ increased turnover associated with growth.14 Sleep is a unique window of opportunity to restore cellular health.23 Even if some biological mechanisms underlying the association between sleep and LTL have been proposed, they are still unclear and need to be elucidated. For example, sleep is related to changes in the immune system through the sleep-wake cycle and disruption in this cycle has been associated with higher inflammation. The latter increases the circulation of proinflammatory cytokines, which may affect the telomere length.23,24 In the same line, changes in cortisol secretion and melatonin have also been linked to telomere length variation through higher oxidative stress.25,26 Hence, it may be hypothesised that a reduced stress environment, lower inflammation and/or oxidation6,9,27 in children who slept more hours per day may be the potential mechanisms that might explain longer telomeres at 4 years in our study. Nonetheless, the complex biology of telomeres—influenced by environmental and genetic factors—makes the investigation in this field very challenging. Therefore, future studies still need to elucidate which biological pathways might explain the association between sleep duration and LTL.
Sleep disturbance and its role in telomere length have been more widely investigated in adults.6,7,8 Thus far, few studies have explored this association in children. James et al. investigated the cross-sectional association between telomere length and sleep duration at age 9 from 1567 children of the Fragile Families and Child Wellbeing Study (a population-based birth cohort of children born between 1998 and 2000 in American cities).12 According to this study, each hour less sleep was associated with 0.015 log-kilobase shorter telomeres, i.e., children with fewer sleep hours had shorter telomeres than those who slept longer.12 Inconsistently, Nguyen et al. showed no evidence for the association between sleep duration—objectively measured—and telomere length in blood in adolescents of 11–12 years from the Longitudinal Study of Australian Children (β = 0.01, 95% CI: –0.04 to 0.06).13 The latter might be explained by the age of the participants since, as it has been proposed, the rate of telomere loss becomes more stable later in life compared with the first years.14
Short sleep duration is a modifiable risk factor contributing to non-communicable diseases such as type 2 diabetes, hypertension and obesity in children.28,29,30 The overuse of technology and screen time has disturbed many children’s sleep hygiene, especially nighttime sleep.31 Sleep routines provide security and help with activity transitions in children and moderate impulsivity.2,31,32 A previous systematic review of approaches to assist in sleep hygiene summarised that using positive routines, controlled comforting and gradual extinction or sleep remodelling are some recommended techniques.33 Given the sleep benefits, consistent bedtime routines and adequate sleep should be highly encouraged to promote positive child development because they may also be associated with a longer telomere length, as disclosed in this study.
Strengths and limitations
This study leveraged data from the INMA birth cohort study, a pioneer project in Spain investigating the role of environmental factors during pregnancy and the beginning of life on growth and development. LTL was objectively measured following standard methods by trained professionals. In addition, we were able to adjust our analyses for an extensive range of confounder factors (both in the primary and sensitivity analyses), including data collected during pregnancy, at birth and during the 4-year follow-up interview. However, this study is not exempt from limitations. Firstly, although children from the INMA project were from different Spain areas, they may not represent the Spanish children population; therefore, estimates should not be fully generalised. Secondly, due to the observational nature of this study, causality cannot be inferred. Nonetheless, the prospective design of the INMA project allows verifying long-term effects in follow-up assessments and identifying potential aetiological factors of disturbances of normal child development over time, thereby establishing a temporal sequence of events. Thirdly, recall bias is possible with self-reported data, as it was the sleep data in this study. Nonetheless, any inaccuracy should be understood as non-differential. Fourthly, LTL was measured using PCR, which shows a higher technical variability than, e.g., terminal restriction fragment (TRF) analysis. However, in large cross-sectional settings, as assessed by qPCR, LTL may be in line with TRF estimated LTL.34 Yet, among the limitations of the PCR method are that it does not provide absolute LTL measures as well as issues detecting very short telomeres or telomeric losses. Therefore, even if a large amount of epidemiological research has conducted their investigation on LTL using the PCR approach,35 findings should be interpreted with caution and telomere dynamics should be confirmed in future longitudinal-based studies. Finally, unmeasured or residual confounding is possible even if we included a long list of confounder factors. Moreover, traumatic events,10,11 a risk factor widely investigated and associated with telomere length, were not included as confounder factors since there was no available information.
In conclusion, children that slept more h/day had a longer LTL at 4 years independently of a wide range of confounder factors. No significant differences were identified in LTL at 7–9 years. Therefore, sleep routines are encouraged to promote positive child development. Yet, considering the complex biology of telomere length, future studies still need to elucidate which biological pathways might explain the association between sleep duration and telomere length.
Data availability
The data that support the findings of this study are not available for sharing due to ethical and legal restrictions implemented by the regional Ethical Committees and the Ethical Committee of the General Hospital of Alicante. As stated in the informed consent form from participants, we guaranteed the confidentiality of collected personal information from questionnaires and related data. Requests to access the data should be submitted to the corresponding author. Requests will be reviewed by the research team and will require a data transfer agreement.
References
Frank, M. G. & Heller, H. C. The function(s) of sleep. Handb. Exp. Pharm. 253, 3–34 (2019).
Mindell, J. A. & Williamson, A. A. Benefits of a bedtime routine in young children: sleep, development, and beyond. Sleep. Med. Rev. 40, 93–108 (2018).
Paruthi, S. et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J. Clin. Sleep. Med. 12, 785–786 (2016).
Wheaton, A. G. & Claussen, A. H. Short sleep duration among infants, children, and adolescents aged 4 months-17 years – United States, 2016-2018. MMWR Morb. Mortal. Wkly Rep. 70, 1315–1321 (2021).
de Ruiter, I., Olmedo-Requena, R., Sánchez-Cruz, J. J. & Jiménez-Moleón, J. J. Changes in sleep duration in Spanish children aged 2–14 years from 1987 to 2011. Sleep. Med. 21, 145–150 (2016).
Carroll, J. E. et al. Insomnia and telomere length in older adults. Sleep 39, 559–564 (2016).
Huang, P. et al. The association between obstructive sleep apnea and shortened telomere length: a systematic review and meta-analysis. Sleep. Med. 48, 107–112 (2018).
Savolainen, K., Eriksson, J. G., Kajantie, E., Lahti, M. & Räikkönen, K. The history of sleep apnea is associated with shorter leukocyte telomere length: the Helsinki Birth Cohort Study. Sleep. Med. 15, 209–212 (2014).
Rentscher, K. E., Carroll, J. E. & Mitchell, C. Psychosocial stressors and telomere length: a current review of the science. Annu. Rev. Public Health 41, 223–245 (2020).
Deighton, S., Neville, A., Pusch, D. & Dobson, K. Biomarkers of adverse childhood experiences: a scoping review. Psychiatry Res. 269, 719–732 (2018).
Coimbra, B. M., Carvalho, C. M., Moretti, P. N., Mello, M. F. & Belangero, S. I. Stress-related telomere length in children: a systematic review. J. Psychiatr. Res. 92, 47–54 (2017).
James, S. et al. Sleep duration and telomere length in children. J. Pediatr. 187, 247–252.e1 (2017).
Nguyen, M. T. et al. Objectively measured sleep and telomere length in a population-based cohort of children and midlife adults. Sleep 43, zsz200 (2020).
Gorenjak, V., Petrelis, A. M., Stathopoulou, M. G. & Visvikis-Siest, S. Telomere length determinants in childhood. Clin. Chem. Lab Med. 58, 162–177 (2020).
Benetos, A. et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12, 615–621 (2013).
Guxens, M. et al. Cohort profile: the INMA–INfancia y Medio Ambiente–(Environment and Childhood) Project. Int J. Epidemiol. 41, 930–940 (2012).
Martens, D. S. et al. Newborn telomere length predicts later life telomere length: tracking telomere length from birth to child- and adulthood. eBioMedicine 63, 103164 (2021).
Martens, D. S. et al. Association of parental socioeconomic status and newborn telomere length. JAMA Netw. Open 3, e204057 (2020).
Notario-Barandiaran, L. et al. High adherence to a mediterranean diet at age 4 reduces overweight, obesity and abdominal obesity incidence in children at the age of 8. Int J. Obes. (Lond.) 44, 1906–1917 (2020).
Buckland, G. et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am. J. Epidemiol. 170, 1518–1529 (2009).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Mickey, R. M. & Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 129, 125–137 (1989).
Cribbet, M. R. et al. Cellular aging and restorative processes: subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 37, 65–70 (2014).
Redwine, L., Dang, J. & Irwin, M. Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav. Immun. 18, 333–340 (2004).
Prather, A. A. et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J. Aging Res. 2011, 721390 (2011).
Liang, G. et al. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS One 6, e23462 (2011).
Prather, A. A. et al. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun. 47, 155–162 (2015).
Dutil, C. & Chaput, J. P. Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutr. Diabetes 7, e266 (2017).
Sluggett, L., Wagner, S. L. & Harris, R. L. Sleep duration and obesity in children and adolescents. Can. J. Diabetes 43, 146–152 (2019).
DelRosso, L. M., Mogavero, M. P. & Ferri, R. Effect of sleep disorders on blood pressure and hypertension in children. Curr. Hypertens. Rep. 22, 88 (2020).
Bathory, E. & Tomopoulos, S. Sleep regulation, physiology and development, sleep duration and patterns, and sleep hygiene in infants, toddlers, and preschool-age children. Curr. Probl. Pediatr. Adolesc. Health Care 47, 29–42 (2017).
Galland, B. C., Taylor, B. J., Elder, D. E. & Herbison, P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep. Med. Rev. 16, 213–222 (2012).
Halal, C. S. & Nunes, M. L. Education in children’s sleep hygiene: which approaches are effective? A systematic review. J. Pediatr. (Rio J.) 90, 449–456 (2014).
Nettle, D. et al. Measurement of telomere length for longitudinal analysis: implications of assay precision. Am. J. Epidemiol. 190, 1406–1413 (2021).
Lai, T. P., Wright, W. E. & Shay, J. W. Comparison of telomere length measurement methods. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20160451 (2018).
Funding
This study was funded by Instituto de Salud Carlos III/Agencia Estatal de Investigación, grant number PI18/00825 Project: ‘Dieta y actividad física en embarazo y tras el nacimiento y longitud del telómero en niños y adolescentes: Proyecto TeloDiPA’ and Unión Europea (FEDER) ‘Una manera de hacer Europa’; Generalitat Valenciana (GVA/2021/191); D.S.M. holds a postdoctoral grant by the Flemish Scientific Fund (FWO grant 12X9620N). In Sabadell, this study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151 incl. FEDER funds; PI12/01890 incl. FEDER funds; CP13/00054 incl. FEDER funds, PI15/00118 incl. FEDER funds, CPII18/00018), CIBERESP, Generalitat de Catalunya-CIRIT 1999SGR 00241, Generalitat de Catalunya-AGAUR (2009 SGR 501, 2014 SGR 822), Fundació La marató de TV3 (090430), Spanish Ministry of Economy and Competitiveness (SAF2012-32991 incl. FEDER funds), Agence Nationale de Securite Sanitaire de l’Alimentation de l’Environnement et du Travail (1262C0010), EU Commission (261357, 308333, 603794 and 634453). We acknowledge support from the Spanish Ministry of Science and Innovation and the State Research Agency through the ‘Centro de Excelencia Severo Ochoa 2019–2023’ Program (CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program. In Asturias, this study was funded by ISCIII: PI04/2018, PI09/02311, PI13/02429, PI18/00909 co-funded by FEDER, ‘A way to make Europe’/‘Investing in your future’, Obra Social Cajastur/Fundación Liberbank and Universidad de Oviedo. In Valencia, this study was supported by grants from Instituto de Salud Carlos III [FIS-FEDER: 13/1944, 16/1288, 19/1338; Miguel Servet-FSE: MS15/0025, MSII20/0006 incl. FEDER funds], Generalitat Valenciana [AICO/2020/285] and Gobierno de España through Ministerio de Universidades under the grant CAS21/00008. The funders had no role in the design of the study; collection, management, analysis and interpretation of the data; preparation, review or approval and submission of the manuscript.
Author information
Authors and Affiliations
Contributions
F.P.-R., D.V.-G. and E.-M.N.-M. contributed to the conception and design of the study, advised on all statistical aspects and interpreted the data. F.P.-R. performed the literature search and the analyses. All authors critically reviewed this and previous drafts. All authors approved the final draft for submission, with final responsibility for publication. E.-M.N.-M. is the guarantor.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The regional Ethical Committees approved the INMA birth cohort study. This study complies with the Helsinki Declaration for human studies. All parents/legal tutors provided their written consent at each phase of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petermann-Rocha, F., Valera-Gran, D., Fernández-Pires, P. et al. Children who sleep more may have longer telomeres: evidence from a longitudinal population study in Spain. Pediatr Res 93, 1419–1424 (2023). https://doi.org/10.1038/s41390-022-02255-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02255-w
This article is cited by
-
Association between telomere length and neuropsychological function at 4–5 years in children from the INMA project: a cross-sectional study
European Child & Adolescent Psychiatry (2024)