Abstract
Background
Cooling delays, temperature outside 33–34 °C, and blood pressure below the mean arterial blood pressure with optimal cerebral autoregulation (MAPOPT) might diminish neuroprotection from therapeutic hypothermia in neonates with hypoxic–ischemic encephalopathy (HIE). We hypothesized that longer time to reach temperature <34 °C and having temperature outside 33–34 °C would be associated with worse autoregulation and greater brain injury.
Methods
Neonates with HIE had rectal temperature and near-infrared spectroscopy autoregulation monitoring during hypothermia (n = 63) and rewarming (n = 58). All underwent brain MRI, and a subset received diffusion tensor imaging MRI before day 10 (n = 41).
Results
Most neonates reached <34 °C at 3–6 h of life. MAPOPT was identified in 54/63 (86%) during hypothermia and in 53/58 (91%) during rewarming. Cooling time was not related to blood pressure deviation from MAPOPT. Later cooling was associated with lower ADC scalar in unilateral posterior centrum semiovale but not in other regions. Temperatures >34 °C were associated with blood pressure above MAPOPT but not with brain injury.
Conclusions
In neonates who were predominantly cooled after 3 h, cooling time was not associated with autoregulation or overall brain injury. Blood pressure deviation above MAPOPT was associated with temperature >34 °C. Additional studies are needed in a more heterogeneous population.
Impact
-
Cooling time to reach target hypothermia temperature within 6 h of birth did not affect cerebral autoregulation measured by NIRS in neonates with hypoxic–ischemic encephalopathy (HIE).
-
Temperature fluctuations >33–34 °C were associated with blood pressures that exceeded the range of optimal autoregulatory vasoreactivity.
-
Cooling time within 6 h of birth and temperatures >33–34 °C were not associated with qualitative brain injury on MRI.
-
Regional apparent diffusion coefficient scalars on diffusion tensor imaging MRI were not appreciably affected by cooling time or temperature >33–34 °C.
-
Additional research in a larger and more heterogeneous population is needed to determine how delayed cooling and temperatures beyond the target hypothermia range affect autoregulation and brain injury.
Similar content being viewed by others
Introduction
Neonatal encephalopathy causes major morbidity and mortality worldwide. Moderate-to-severe hypoxic–ischemic encephalopathy (HIE) is a major cause of neonatal encephalopathy and affects approximately 1.5–3 per 1000 live term births in developed countries.1 Initiating therapeutic hypothermia (TH) within 6 h of birth provides only partial neuroprotection, with 35–55% of survivors having persistent neurologic disabilities at age 6–7 years.2,3 The risk of HIE and poor outcome is even higher in regions with limited access to early and rapid cooling.4
Brain injury from hypoxic–ischemia (HI) evolves from the primary injury to transient recovery and is followed by a latent phase over approximately 6 h.5 TH to a core temperature of 33–34 °C for 72 h should be initiated within 6 h of birth to reduce injury from subsequent secondary energy failure.6 The time that passes before the goal temperature is reached and temperature deviations from 33–34 °C may affect therapeutic efficacy. Though immediate TH after moderate HI is neuroprotective in rat pups, this protection declines linearly if cooling is delayed by 3–6 h.7 Clinically, neonates who receive hypothermia within 1 h after birth have fewer seizures and shorter hospitalizations than do neonates cooled after the first hour of life.8 Motor neurodevelopmental outcomes are also improved by initiating TH within 3 h of birth.9 Thus cooling delays, even within 6 h of birth, and failure to remain within 33–34 °C might reduce neuroprotection.
Dysfunctional cerebrovascular autoregulation and vasoreactivity are also associated with greater brain injury and worse neurodevelopmental outcomes after HIE.10,11,12,13,14,15,16,17 Autoregulation holds cerebral blood flow relatively constant across changes in perfusion pressure through changes in cerebrovascular resistance. The range of blood pressure with most robust vasoreactivity is the optimal mean arterial blood pressure (MAPOPT), and greater blood pressure deviation from MAPOPT indicates dysfunctional autoregulation. Several studies have shown that blood pressure deviation below MAPOPT is associated with greater neurologic injury in neonates with HIE.10,11,12,13,17
In this pilot study, we examined whether time to achieve the target TH temperature (i.e., cooling time) and temperature deviation outside 33–34 °C are associated with disturbed autoregulation and early brain injury in neonates with HIE. Because perinatal insults affect neurologic injury, we used a perinatal insult score to grade the neonate’s clinical condition.11 We tested the primary hypothesis that longer cooling time to achieve <34 °C is associated with greater blood pressure deviation from MAPOPT and greater brain injury on magnetic resonance imaging (MRI) when controlling for perinatal insults. We secondarily tested whether temperature fluctuations outside 33–34 °C are associated with worse autoregulation and brain injury.
Methods
The Johns Hopkins Medicine Institutional Review Board (IRB) approved this prospective, observational study. Written informed consent was obtained through May 2013; thereafter, the IRB granted a waiver of consent because near-infrared spectroscopy (NIRS) became standard clinical care during TH for neonates with HIE at Johns Hopkins Hospital (JHH).
Subjects
HIE was diagnosed according to criteria of the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network.18 All neonates initially diagnosed with moderate-to-severe HIE were screened upon admission to the JHH neonatal intensive care unit (NICU) from September 2010 to July 2015. Eligible infants had gestational age ≥35 weeks, arterial blood pressure monitoring, TH, and additional eligibility criteria that we have detailed previously.10,13 All neonates in the current study were also reported in our past autoregulation studies.10,11,12,13,17,19,20
Protocol and clinical care
Neonates were cooled to a goal core temperature of 33.5 ± 0.5 °C for 72 h and then rewarmed to a rectal temperature of 36.5 °C at a rate of 0.5 °C/h.13 Neonates inborn at JHH were actively cooled upon NICU admission, whereas outborn neonates from regional hospitals were passively cooled during transport to JHH. The transport team recorded the time that a newborn’s core temperature reached <34 °C if it was achieved by passive cooling alone. No outborn neonate received active cooling during transport.
Cooling time (h) was calculated as the difference between the time of first rectal temperature <34 °C and time of birth. Hourly rectal temperatures, medications, presence of seizures, and blood gas data were obtained from the electronic medical record. Using common clinical criteria (Table 1), we assigned a perinatal insult score to describe the neonates’ clinical status soon after birth.11 We developed this score previously to provide an assessment of insult severity and generate a clinical description variable for multivariate analysis.
Autoregulation monitoring
Arterial blood pressure and bilateral cerebral NIRS (neonatal probes; INVOS 5100; Medtronic, Minneapolis, MN) were monitored continuously in the neonates, enabling us to calculate the hemoglobin volume index (HVx) as a continuous measure of cerebral vasoreactivity according to our published methods.13,21,22 Briefly, the NIRS relative total tissue hemoglobin (rTHb) is a surrogate measure of cerebral blood volume. Autoregulatory vasoreactivity causes slow wave changes in cerebral blood volume that are detected by rTHb as the blood pressure fluctuates.21,22 HVx is generated by correlating MAP and rTHb. When vasoreactivity is functional, HVx is negative and approaches −1 because MAP and rTHb negatively correlate. HVx becomes positive and approaches +1 when vasoreactivity is dysfunctional, with pressure-passive MAP and rTHb positively correlated. The 5-mm Hg blood pressure range with the most negative HVx (nadir) is the MAPOPT at which autoregulatory vasoreactivity is most robust.13,17 Neonates without an apparent HVx nadir were coded as having an unidentifiable MAPOPT.
We measured autoregulatory vasoreactivity during hypothermia and rewarming using (1) the percentage of the monitoring period spent with blood pressure below, within, or above MAPOPT; (2) the maximal blood pressure deviation below or above MAPOPT; and (3) the area under the curve (AUC; min*mm Hg/h) of time (min) with blood pressure below MAPOPT and blood pressure deviation (mm Hg) below MAPOPT normalized to the monitoring duration (h).11
Brain MRI
Neonates received T1, T2, and diffusion tensor imaging (DTI) MRIs between 4 and 16 days from birth on a 1.5 T (Avanto) or 3 T (Siemens) clinical scanner (Erlangen, Germany) during natural sleep without anesthesia. We previously reported our MRI methods.10,11 Qualitative brain injury was scored as none, mild, moderate, or severe in six regions (paracentral gyri, global white matter, basal ganglia, thalamus, posterior limb of the internal capsule (PLIC), and brainstem) in all neonates. We previously published the qualitative injury data in this cohort.11
Apparent diffusion coefficient (ADC) scalars were measured as the median of three region-of-interest measurements in the anterior and posterior centrum semiovale, PLIC, basal ganglia, thalamus, pons, and cerebellar white matter. Right and left sides were analyzed separately in all areas except for the pons. We analyzed DTI data only in neonates who received their MRI before day 10 of life to account for pseudonormalization of the ADC.21 We previously published the DTI data collected by the 1.5 T scanner.10,12
Statistical analysis
Cooling time was analyzed in two ways. First, we analyzed the time to reach core temperature <34 °C as a dichotomous variable (<3 h or ≥3 h after birth) based on evidence that motor neurodevelopmental outcomes are improved if cooling is initiated within 3 h of birth.9 We also analyzed cooling time as a continuous measure.
After the neonate reached rectal temperature <34 °C, the temperature AUC (degree*min/h) was calculated as any deviation above or separately below 33–34 °C during 72 h of TH. For example, a neonate at 35 °C for 1 h would have an AUC of 60 degree*min/h. Having a temperature of 34.5 °C for 1 h gives an AUC of 30 degree*min/h.
For univariate analyses, logistic and linear regressions were used to estimate the associations between cooling time or temperature AUC and the outcomes for blood pressure relative to MAPOPT (percentage of the monitoring period with blood pressure below, within, or above MAPOPT; maximal blood pressure deviation below or above MAPOPT; and AUC of blood pressure below MAPOPT) during hypothermia and separately during rewarming. We used ordered logistic regression (for categorical measurements), assuming proportional odds and linear regressions, to estimate the associations between cooling time, temperature AUC, and perinatal insult score with the outcomes for regional qualitative (categorical) brain injury in all neonates and DTI MRI in neonates who received scans before 10 days of age. MRI analyses were corrected for multiple comparisons.
In addition, we adjusted the analyses by the perinatal insult score and the partial pressure of carbon dioxide (PaCO2) during hypothermia and rewarming. The degree of perinatal insult may independently affect autoregulation, blood pressure regulation around MAPOPT, and brain injury. PaCO2 <35 mm Hg is also an independent risk factor for brain injury during HIE,23 and PaCO2 influences autoregulation.24 We placed each neonate’s PaCO2 during hypothermia and rewarming into one of the four categories: (1) all PaCO2 levels 35–45 mm Hg; (2) all <45 mm Hg and some <35 mm Hg; (3) all >35 mm Hg and some >45 mm Hg; or (4) some <35 mm Hg and some >45 mm Hg.11
Differences in clinical characteristics between the entire cohort and neonates who received DTI MRI prior to the tenth day of life were analyzed by chi square test, Student’s t test, or Fisher’s exact test as appropriate. Finally, the cooling time for inborn and outborn neonates was compared by Mann–Whitney test. A p < 0.05 was considered statistically significant.
Sample size calculation
Limited information is available about the effects of delayed clinical cooling and temperature fluctuations on autoregulation of neonates during TH for HIE. Because we did not have a priori data about the potential associations between cooling time or temperature deviation from 33–34 °C and autoregulatory vasoreactivity or region-specific brain injury on MRI, we did not conduct sample size or power estimates for this exploratory, pilot study.
Results
Among 122 screened neonates with HIE, 75 had HVx monitoring (Fig. 1). Sixty-four neonates with brain MRI data had HVx monitoring during hypothermia (mean monitoring duration 46.5 h [SD, 19.8]), and 59 were monitored during rewarming (mean duration 6.3 h [SD, 2.6]). One outborn neonate who experienced extreme delays in HIE diagnosis and transport to the JHH NICU was excluded because the goal temperature was not achieved until 10 h after birth. Thus 63 neonates were included in the study. MAPOPT was identified in 54 of the 63 neonates (86%) during hypothermia and in 53 of the 58 (91%) during rewarming. We analyzed all neonates’ qualitative brain injury on MRI. ADC measures were analyzed in 41 neonates who received DTI MRI before 10 days of age. Owing to limitations related to changes in the electronic medical record system twice at JHH, hourly rectal temperatures to calculate temperature AUC outside 33–34 °C were available in only 51 of the 63 neonates (81%) with qualitative brain injury measures on MRI and in 35 of the 41 (85%) with DTI MRI.
Clinical description
The mean gestational age was 38 weeks and 5 days (SD 1.5; n = 63) with 47 of the 63 (75%) born by cesarean section, and 59% were males (Table 2). In addition, the majority were outborn, diagnosed with moderate HIE (Sarnat score 2), and reached a core temperature <34 °C after 3 h from birth. Three newborns diagnosed with moderate HIE at an outside hospital were passively cooled before and during transport. Their Sarnat scores of 1 were determined during hypothermia after arrival to JHH. Because these newborns were initially diagnosed with moderate HIE and they met our institutional criteria for cooling,13 the clinical team continued the TH protocol.
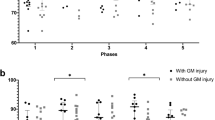
Outborn neonates transported to the JHH NICU had a mean cooling time of 4.4 h (SD: 1.3; n = 53), whereas those inborn were cooled by 1.7 h (SD, 0.9; n = 10; p < 0.001; Fig. 2). Temperature was rarely <33 °C (Table 2) and the mean was 33.5 °C (SD, 0.79) during 72 h of TH. The subset of neonates with DTI MRI had temperature outside 33–34 °C more often than the entire cohort (p = 0.015) with the majority of these temperatures exceeding the target (p = 0.027). MAP ranged from 30 to 75 mm Hg during hypothermia and rewarming.11
Inborn neonates reached the goal core temperature faster than did outborn neonates (*p < 0.001). Though cooling began within 6 h of birth for all neonates, outborn neonates were passively cooled during transport, whereas inborn neonates received early active cooling. Medians with interquartile ranges are shown. Each circle represents one neonate.
Qualitative brain injury measures on MRI showed that most neonates had no or mild injury in the paracentral gyri, white matter, basal ganglia, thalamus, PLIC, and brainstem (Supplementary Table S1). Moderate or severe injury was most common in the white matter (35%) and thalamus (30%), followed by brainstem (26%) and basal ganglia (25%).
Electroencephalographic seizures were identified in 24 of the 63 neonates (38%) during hypothermia and rewarming, and all received phenobarbital. Nine (14%) additionally received leviteracetam, 2 (3%) received fosphenytoin, and 1 (1.5%) received topiramate. Forty-two (67%) were administered dopamine, 12 (19%) dobutamine, 5 (8%) epinephrine, and 6 (10%) milrinone. Hydrocortisone was administered for hypotension refractory to inotropes in 11 (18%). Sedation was provided with morphine (63; 100%) per the clinical TH protocol, plus fentanyl (6; 10%), hydromorphone (3; 5%), clonidine (9; 14%), or benzodiazepines (7; 11%) as needed clinically.
Perinatal insult score
Higher perinatal insult score was associated with greater qualitative MRI injury in the paracentral gyri in univariate analysis (β = 1.77; 95% confidence interval [CI]: 1.15, 2.27; p = 0.012; n = 63) and after adjusting for PaCO2 (β = 1.83; 95% CI: 1.16, 2.87; p = 0.012). Higher perinatal insult score was also associated with greater microstructural injury in the left posterior centrum semiovale by DTI MRI when controlling for PaCO2 (β = −31.7; 95% CI: −61.2, −2.3; p = 0.042; n = 41).
Cooling time
Cooling time <3 h or ≥3 h or as a continuous measure was not associated with MAPOPT or blood pressure relative to MAPOPT during hypothermia or rewarming in univariate analyses nor after adjustment for the perinatal insult score and PaCO2 (Supplementary Tables S2, S3). Moreover, cooling time <3 h or ≥3 h or as a continuous measure was not related to regional qualitative brain injury by MRI in univariate or multivariate analyses (n = 63; Supplementary Tables S4, S5).
Among the 41 neonates with DTI MRI, those cooled to <34 °C at ≥3 h had lower ADC scalars in the right posterior centrum semiovale by univariate analysis (p = 0.036) and after adjustment for the perinatal insult score and PaCO2 (p = 0.035) when compared to neonates cooled earlier (Table 3). No other regional ADC scalars were associated with cooling time in univariate or multivariate analyses.
Temperature deviation from 33–34 °C
Greater temperature AUC >34 °C was associated with more blood pressure deviation above MAPOPT during hypothermia after we adjusted for the perinatal insult score and PaCO2 (p = 0.041; Table 4). Similarly, during rewarming, greater temperature AUC >34 °C related to greater time with blood pressure above MAPOPT (p = 0.013) and less time with blood pressure below MAPOPT (p = 0.027) in adjusted comparisons. Neonates rarely had temperature <33 °C (Table 2). Temperature >34 °C was not associated with regional qualitative brain injury (n = 51) or ADC scalar (n = 35) in univariate or multivariate analyses (Supplementary Tables S6 and S7).
Discussion
Rapid cooling of core temperature and maintenance at 33–34 °C are keys to effective TH when treating neonates with HIE. However, achieving these metrics can be clinically challenging. We tested whether the time to reach temperature <34 °C and deviation from 33–34 °C were related to blood pressure relative to MAPOPT or early MRI brain injury in a single-center pilot study. Higher MAPOPT and blood pressure deviation from MAPOPT indicate disturbed autoregulatory vasoreactivity. In our cohort, most neonates were outborn, cooled at 3–6 h of life, and had moderate HIE with relatively mild brain injury on MRI. We did not identify a relationship between cooling time, MAPOPT, and blood pressure relative to MAPOPT. Longer time to reach core temperature <34 °C was associated with lower ADC in unilateral posterior centrum semiovale but not in other brain regions after we adjusted for the perinatal insult score and PaCO2. Cooling time had no relationship to qualitative brain injury on MRI. Temperature fluctuation >34 °C was associated with blood pressure above MAPOPT without an effect on brain injury. Neonates rarely had temperatures <33–34 °C. While our pilot data suggest that cooling time and deviation from 33–34 °C minimally impact autoregulation and early brain injury, additional studies are needed in a larger and more heterogeneous population.
Immediate hypothermia reduces cytotoxic edema in patients with HIE.25 In addition, greater cytotoxic edema with lower MRI ADC scalars26 is associated with higher MAPOPT in neonates with HIE.12 This relationship suggests that severe cytotoxic edema might limit cerebral vasodilatory capacity at low blood pressures, thereby shifting MAPOPT to a higher pressure with a rightward shift in the autoregulation curve. Because blood pressure below MAPOPT is associated with greater neurologic injury,10,11,13,17 we theorized that faster cooling and strict adherence to 33–34 °C might reduce cytotoxic edema on DTI MRI, improve blood pressure regulation to be within or close to MAPOPT, and decrease neurologic injury.
Overall, we did not identify an association between cooling time or temperature fluctuations from 33–34 °C and brain injury. Certainly this lack of association does not negate the importance of early cooling with vigilant temperature control. Clinical and preclinical studies clearly show that cooling must begin early and within 6 h of birth.7,9,25,27 All of our neonates were appropriately cooled within 6 h and had a sustained mean core temperature of 33.5 °C (SD, 0.79) for 72 h of TH. Our findings may be related to our patient sample being small with limited variability in cooling time owing to the predominance of outborn neonates. However, our findings are in agreement with a recent study that also showed no relationship between early cooling and MRI brain injury.28 Though lower ADC scalars in unilateral posterior centrum semiovale were associated with later cooling after we adjusted for the perinatal insult score and PaCO2, we did not identify any other relationships between temperature and brain injury.
Numerous factors could delay hypothermia induction, including resuscitation, delays in diagnosing moderate or severe HIE, prolonged transport, agitation, or shivering. Some outborn neonates must be passively cooled at a slower rate as not all NICU transport teams may have active cooling devices. Accordingly, our predominantly outborn population reached <34 °C after 3 h. We could not directly study the independent effects of inborn or outborn status because too few neonates were inborn.
Greater temperature deviation >34 °C during TH was associated with having blood pressure above MAPOPT during both hypothermia and rewarming after adjustment for PaCO2 and the perinatal insult score. Maximal MAP was 75 mm Hg, as previously reported for this cohort.11 Blood pressure above MAPOPT is related to lesser paracentral gyri injury in neonates with HIE11 and lower risk of intraventricular hemorrhage in prematurity.29 Accordingly, temperature >34 °C with blood pressure above MAPOPT showed no association with early MRI brain injury in the current study. Given the risk of hyperemia, we do not advocate intentionally raising the blood pressure above typical clinical goal ranges.
We created the perinatal insult score11 to describe the severity of a neonate’s clinical condition in a single metric for multivariate analysis. Higher perinatal insult score was associated with greater paracentral gyri and posterior centrum semiovale injury. Though we adjusted our analyses using this score, we still cannot distinguish whether dysfunctional autoregulation causes secondary neurologic injury or whether severe brain injury causes dysfunctional autoregulation with blood pressure deviation from MAPOPT.
We analyzed different regions of interest rather than using a global MRI score because HIE vulnerability varies by brain region.30,31,32,33 Higher MAPOPT from rightward shifts in the autoregulation curve are associated with injury detected by regional DTI but not all global MRI scores, such as the NICHD Neonatal Research Network score.12 It is also critical to examine how NIRS autoregulation monitoring in frontal cortex relates to potential injury in more distal regions.
Most of our neonates had moderate HIE with limited variation in brain injury severity. Three were initially diagnosed with moderate encephalopathy at an outside hospital where passive cooling was initiated. Their Sarnat scores were subsequently assessed to be 1 after admission to the JHH NICU for active cooling. Some neonatologists initiate TH for milder HIE given that encephalopathy can evolve rapidly.34 Moreover, perinatal acidosis predicts persistent neurologic injury even if the newborn is classified as having mild encephalopathy.35 At our institution, these three newborns met criteria for TH and were thus enrolled in the study. It is possible that cooling time and temperature deviation from 33–34 °C could have stronger influence on autoregulation and brain injury in neonates with more severe HIE.
We adjusted the analyses for the perinatal insult score11 and PaCO2 because birth injury and PaCO2 are known to affect subsequent brain injury23 and autoregulation24 and may confound time to cooling. Hypercarbia can shift the autoregulation curve,24 and hypocarbia is independently associated with poor neurologic outcome in those with HIE.36 With the exception of a single association between longer time to goal temperature and lower unilateral ADC in posterior centrum semiovale, we found no relationships between cooling time, autoregulation, and brain injury in univariate and multivariate analyses. Temperature deviation >34 °C was also not associated with brain injury. Therefore, we did not adjust for other factors in the statistical modeling, such as vasoactive medications, seizures, or sex.
Limitations
This study was conducted in a single, university-based NICU, thus findings may not be generalizable to other centers. Without a priori data about cooling time, temperature deviation from 33–34 °C, and autoregulation, we could not conduct a sample size estimate. We designed this exploratory pilot study to generate potential hypotheses for future studies. However, the small sample size of neonates cooled before 3 h may have left us underpowered to detect true differences. Our neonates had predominantly moderate HIE and relatively mild brain injury on MRI. Additional studies in a larger and more heterogeneous population with more neonates cooled earlier and greater brain injury variability are needed. Larger studies will also enable analytic adjustments for potential confounders, such as inborn or outborn status. We did not study clinical care at the birth hospital and during transport, which may influence the risk of brain injury. Many neonates with HIE were excluded owing to study ineligibility and other factors described in Fig. 1. Selection bias cannot be ruled out. Finally, we analyzed hourly rectal temperature data, because continuous temperature recordings were not available.
Conclusion
In a population of predominantly outborn neonates cooled to <34 °C at 3–6 h of life for moderate HIE, cooling time did not affect autoregulation or overall early brain injury on MRI. Longer time to reach core temperature <34 °C was associated with lower ADC in unilateral posterior centrum semiovale but not in other brain regions. Temperature deviation >34 °C was related to blood pressure exceeding MAPOPT but did not affect brain injury. These pilot study findings do not negate the importance of rapid cooling with vigilant temperature control. Additional studies are needed in a larger and more heterogeneous population.
References
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Azzopardi, D. et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 371, 140–149 (2014).
Kali, G. T., Martinez-Biarge, M., Van Zyl, J., Smith, J. & Rutherford, M. Management of therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy in a tertiary centre in South Africa. Arch. Dis. Child. Fetal Neonatal Ed. 100, F519–F523 (2015).
Gunn, A. J. & Thoresen, M. Hypothermic neuroprotection. NeuroRx 3, 154–169 (2006).
Wassink, G., Gunn, E. R., Drury, P. P., Bennet, L. & Gunn, A. J. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 8, 40 (2014).
Sabir, H., Scull-Brown, E., Liu, X. & Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 h in neonatal rats. Stroke 43, 3364–3370 (2012).
Youn, Y. A. et al. The hospital outcomes compared between the early and late hypothermia-treated groups in neonates. J. Matern. Fetal Neonatal Med. 29, 2288–2292 (2016).
Thoresen, M. et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 104, 228–233 (2013).
Tekes, A. et al. Apparent diffusion coefficient scalars correlate with near-infrared spectroscopy markers of cerebrovascular autoregulation in neonates cooled for perinatal hypoxic-ischemic injury. AJNR Am. J. Neuroradiol. 36, 188–193 (2015).
Lee, J. K. et al. Optimizing cerebral autoregulation may decrease neonatal regional hypoxic-ischemic brain injury. Dev. Neurosci. 39, 248–256 (2017).
Carrasco, M. et al. Cerebral autoregulation and conventional and diffusion tensor imaging magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. Pediatr. Neurol. 82, 36–43 (2018).
Howlett, J. A. et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr. Res. 74, 525–535 (2013).
Massaro, A. N. et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J. Neurophysiol. 114, 818–824 (2015).
Vesoulis, Z. A., Liao, S. M. & Mathur, A. M. Late failure of cerebral autoregulation in hypoxic-ischemic encephalopathy is associated with brain injury: a pilot study. Physiol. Meas. 39, 125004 (2018).
Tian, F., Tarumi, T., Liu, H., Zhang, R. & Chalak, L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. Neuroimage Clin. 11, 124–132 (2016).
Burton, V. J. et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 15, 209 (2015).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 353, 1574–1584 (2005).
Chavez-Valdez, R. et al. Associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury may be sex-specific in neonates treated with therapeutic hypothermia for hypoxic-ischemic encephalopathy. Pediatr. Res. 81, 759–766 (2017).
Lee, J. K. et al. Relationships between cerebral autoregulation and markers of kidney and liver injury in neonatal encephalopathy and therapeutic hypothermia. J. Perinatol. 37, 938–942 (2017).
Larson, A. C. et al. Cerebrovascular autoregulation after rewarming from hypothermia in a neonatal swine model of asphyxic brain injury. J. Appl. Physiol. 115, 1433–1442 (2013).
Lee, J. K. et al. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40, 1820–1826 (2009).
Pappas, A. et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 158, 752–758.e751 (2011).
Nusbaum, D. M., Brady, K. M., Kibler, K. K. & Blaine Easley, R. Acute hypercarbia increases the lower limit of cerebral blood flow autoregulation in a porcine model. Neurol. Res. 38, 196–204 (2016).
Gunn, A. J., Gunn, T. R., de Haan, H. H., Williams, C. E. & Gluckman, P. D. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Invest. 99, 248–256 (1997).
Bevers, M. B. et al. Apparent diffusion coefficient signal intensity ratio predicts the effect of revascularization on ischemic cerebral edema. Cerebrovasc. Dis. 45, 93–100 (2018).
Roelfsema, V. et al. Window of opportunity of cerebral hypothermia for postischemic white matter injury in the near-term fetal sheep. J. Cereb. Blood Flow Metab. 24, 877–886 (2004).
Guillot, M. et al. Influence of timing of initiation of therapeutic hypothermia on brain MRI and neurodevelopment at 18 months in infants with HIE: a retrospective cohort study. BMJ Paediatr. Open 3, e000442 (2019).
da Costa, C. S., Czosnyka, M., Smielewski, P. & Austin, T. Optimal mean arterial blood pressure in extremely preterm infants within the first 24 h of life. J. Pediatr. 203, 242–248 (2018).
O’Brien, C. E. et al. Hypoxia-ischemia and hypothermia independently and interactively affect neuronal pathology in neonatal piglets with short-term recovery. Dev. Neurosci. 41, 17–33 (2019).
Santos, P. T. et al. Proteasome biology is compromised in white matter after asphyxic cardiac arrest in neonatal piglets. J. Am. Heart Assoc. 7, e009415 (2018).
Wang, B. et al. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience 316, 296–310 (2016).
Wang, B. et al. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 35, 781–793 (2015).
Olsen, S. L. et al. Optimizing therapeutic hypothermia for neonatal encephalopathy. Pediatrics 131, e591–e603 (2013).
DuPont, T. L. et al. Short-term outcomes of newborns with perinatal acidemia who are not eligible for systemic hypothermia therapy. J. Pediatr. 162, 35–41 (2013).
Lopez Laporte, M. A. et al. Association between hypocapnia and ventilation during the first days of life and brain injury in asphyxiated newborns treated with hypothermia. J. Matern. Fetal Neonatal Med. 32, 1312–1320 (2019).
Acknowledgements
We are grateful to Claire Levine, MS, ESL for her editorial assistance. This study was supported by funding from the National Institutes of Health (grant numbers R01NS107417, R01NS109029, and K08NS080984 [to J.K.L.]; K08NS096115 [to R.C.-V.]; R01HD070996 and R01HD086058 [to F.J.N.]; and R01NS107417 [to A.T.]); the American Heart Association Transformational Project Award (co-funded by the Lawrence J. and Florence A. DeGeorge Charitable Trust; 18TPA34170077 [to J.K.L.]); the Johns Hopkins University-School of Medicine Clinician Scientist Award (to R.C.-V.); and the Sutland-Pakula Endowment for Neonatal Research (to R.C.-V.).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: all authors. Drafting the article or revising it critically for important intellectual content: M.M.G., A.T., J.P., R.C.-V., F.J.N., and J.K.L. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
J.K.L., F.J.N., M.M.G., and R.C.-V. received research support from Medtronic for a separate study. J.K.L. was also a paid consultant for Medtronic, and she received research support from Casmed. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Medtronic and Casmed had no role in the design of the current study, collection or analysis of the data, interpretation of the results, manuscript writing, or in our decision to submit this manuscript for publication. A.T., J.P., H.S., and C.P. have nothing to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gilmore, M.M., Tekes, A., Perin, J. et al. Later cooling within 6 h and temperatures outside 33–34 °C are not associated with dysfunctional autoregulation during hypothermia for neonatal encephalopathy. Pediatr Res 89, 223–230 (2021). https://doi.org/10.1038/s41390-020-0876-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0876-8