Abstract
Background
Gestational weight gain (GWG) has been linked to childhood obesity. However, it is unclear if the timing of weight gain influences offspring body composition. A secondary analysis of a clinical trial examined the influence of total, early, and mid-pregnancy GWG on adiposity outcomes in 186 children at birth, 1, 3, and 5 years.
Methods
Early (<15 weeks) and mid-pregnancy GWG (15–32 weeks) were assessed. Anthropometrics and abdominal ultrasound were measured annually in children from birth to 5 years. MRI was performed in a sub-group of 44 children at 5 years to estimate abdominal fat.
Results
Almost half of the women (n = 86/186) gained excess weight in pregnancy, and women with a BMI ≥ 25 kg/m2 (n = 33) were more likely to gain in excess. Mid-pregnancy GWG predicted higher weight (g) and subcutaneous fat by ultrasound (mm2) and MRI (cm3) at 5 years [β: 139.34 g (95% CI: −0.22; 278.90), p = 0.050; β: 1.42 mm2 (95% CI: 0.06; 2.78), p = 0.041; and β: 18.56 cm3 (95% CI: 1.30; 35.82) p = 0.036, respectively].
Conclusions
Mid-pregnancy weight gain was associated with greater fat depots at 5 years, which suggests that the timing of GWG has differential effects on offspring adiposity outcomes.
Impact
-
Gestational weight gained in mid-pregnancy is associated with growth and adipose tissue development at 5 years.
-
We observed that maternal weight gain in early and mid-gestation has differential effects on offspring body composition.
-
Mid-pregnancy weight gain (15–32 weeks gestation) appears to influence child growth and abdominal fat accretion which may have implications for long-term metabolic health.
-
Interventions that prevent excessive gestational weight gain in mid-pregnancy may affect obesity risk in early childhood.
-
Prenatal care should stress the importance of optimal weight gain throughout pregnancy.
Similar content being viewed by others
Introduction
Childhood obesity is a global public health priority. In the past few decades, research has investigated whether its developmental origins begin in utero, where sub-optimal conditions can alter fetal gene expression and lead to long-term metabolic changes.1 Pre-pregnancy body mass index (BMI) and gestational weight gain (GWG) are two prenatal risk factors that have been identified as strong predictors of offspring growth.2 GWG is a critical indicator of the fetal nutritional environment, and research suggests that weight gain above the Institute of Medicine (IOM) recommendations2 is associated with an increased risk of obesity in children.3,4 Most studies focus on total GWG; however, research suggests that weight gained in different gestational windows may have varying effects on offspring adiposity outcomes.5,6
Perinatal research has also examined the role of maternal pre-pregnancy BMI on long-term health in children.2 Previous work has shown that maternal pre-pregnancy overweight/obesity is associated with an increased likelihood of high birth weight, large for gestational age, and later offspring obesity risk.7,8,9
Elevated pre-pregnancy BMI and excess GWG both result in over-nutrition of the fetus, suggesting that their combined effect may amplify adverse child health outcomes. However, most research on prenatal determinants of later obesity have examined maternal BMI and GWG as independent predictors, and it is poorly understood if the two interact to generate a combined effect on offspring growth and development. Studies that have examined the interaction between pre-pregnancy BMI and GWG demonstrate increased adiposity outcomes in early infancy.8,10,11 However, it is unclear if this effect persists into childhood.12,13,14,15
Studies that have investigated the aforementioned associations often report weight or BMI as their primary outcome measure, both of which are poor predictors of adiposity in children.16,17
We took an exploratory research approach in this secondary analysis to investigate associations between GWG (total as well as early and mid-gestation) and offspring body composition at birth, 1, 3, and 5 years of age. Further, we were interested in exploring whether maternal pre-pregnancy BMI interacts with total GWG to influence offspring growth and adipose tissue development. We took advantage of several complementary methods to assess fat accumulation and distribution, including anthropometric indicators of body composition and abdominal ultrasound, as well as performing abdominal magnetic resonance imaging (MRI) in a sub-group of 5-year-old children.
Methods
Study design
This study is embedded in the Impact of Nutritional Fatty acids during pregnancy and lactation on early human Adipose Tissue development (INFAT) study, a randomized controlled clinical trial that examined if reducing the n-6/n-3 long-chain polyunsaturated fatty acids (LCPUFA) ratio in pregnancy and lactation leads to an effect on offspring body composition in the first 5 years of life. Study design and outcomes have been previously reported.18,19,20 In brief, 208 healthy women with a pre-pregnancy BMI between 18 and 30 kg/m2 were recruited before their 15th week of pregnancy. Women were randomly assigned to a control or intervention group. The intervention group received a daily supplement of fish oil capsules containing 1020 mg docosahexaenoic acid + 180 mg eicosapentaenoic acid from 15 weeks’ gestation to 4 months’ postpartum along with dietary advice to achieve an arachidonic acid balanced diet. Women in the control group received standard dietary advice according to current German recommendations. We could not confirm that n-3 LCPUFA supplementation during pregnancy and lactation influenced offspring fat accretion in infancy and early childhood.19,20 This secondary analysis pooled women from the INFAT intervention and control groups to form one cohort.
Data collection
Anthropometric measurements of offspring were recorded by trained investigators at birth and annually thereafter up to the age of 5 years.19,20 BMI percentiles were calculated from German pediatric growth charts.21 Skinfold thickness (SFT) measurements at four body sites (triceps, biceps, subscapular, and suprailiac) were measured in triplicate on the left body axis. Mean readings were used to calculate the sum of the four SFTs. Abdominal adipose tissue was assessed for all children by ultrasound annually.22,23 All families were approached to participate in an abdominal MRI at 5 years. In total, 22 children from the intervention group and 22 children from the control group agreed to participate. Ultrasound and MRI methods are discussed in detail in previous publications.20,22,23 The IOM has developed guidelines for total GWG in singleton pregnancies according to pre-pregnancy BMI.2 Women with normal weight (BMI 18.5–24.9 kg/m2) should gain between 11.5 and 16 kg, women with overweight (BMI 25.0–29.9 kg/m2) between 7 and 11.5 kg, and women with obesity (BMI ≥ 30 kg/m2) between 5 and 9 kg. Pre-pregnancy weight was self-reported. Maternal weight at 15 weeks, 32 weeks, and before delivery were taken from maternity records. Early pregnancy weight gain was defined as the difference between maternal weight at 15 weeks’ gestation and pre-pregnancy weight. Mid-pregnancy weight gain was defined as the difference between maternal weight at 32 weeks’ gestation and 15 weeks’ gestation. Total GWG was calculated as the difference between the last weight recorded before delivery and pre-pregnancy weight. Women were categorized as having gained inadequate, adequate, or excessive total gestational weight during pregnancy according to the IOM guidelines.2
The study was approved by the Technical University of Munich Ethics Committee, Munich, Germany (1478/06/2009/10/26). Written informed consent was obtained from both parents. For MRI scans, the accompanying parent gave written informed consent. The clinical trial is registered at clinicaltrials.gov, number ID NCT00362089, http://clinicaltrials.gov/ct2/show/NCT00362089.
Statistical analysis
Statistical analyses were performed with SPSS version 25 (IBM, New York, NY, USA). All analyses performed were exploratory and no adjustments were made for multiple comparisons. A two-sided p value of ≤0.05 was considered statistically significant.
Total GWG was classified as inadequate, adequate, or excessive according to IOM recommendations per pre-pregnancy BMI category. Differences between women in each total GWG category were compared using one-way ANOVA for continuous variables and Pearson chi-square tests for categorical variables. Interaction models were fit to explore if pre-pregnancy BMI modified the effect of total GWG on child clinical outcomes. To investigate GWG (total, early, and mid-pregnancy) as predictor variables, linear regression models were fit to child clinical characteristics. GWG was examined as a continuous variable for all models. We previously reported that women in the intervention group had a significantly longer gestational period;19 therefore, regression models were adjusted for pregnancy duration (in days). Models were also controlled for maternal pre-pregnancy BMI, study group (intervention or control), and sex (except for BMI percentiles). All child outcomes from 1 year onwards were additionally adjusted for mode of infant feeding at 4 months (exclusively breastfeeding, mixed feeding, or formula only).
Results
From a total of 186 mother/child pairs in the data set, half of the women (49.5%) in the INFAT intervention group and 42.9% of women in the control group gained excess weight in pregnancy, with no statistical difference observed (p = 0.366). Intervention and control groups were pooled for all subsequent analyses.
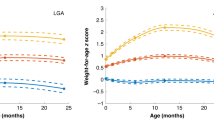
Table 1 shows selected characteristics of mothers and children grouped by total GWG category (inadequate, adequate, and excessive). Women with excess weight gain (46%) began their pregnancies with a significantly higher weight and BMI than women who gained inadequate or adequate weight (p = 0.001, both comparisons). Stratification by BMI category showed that women with pre-pregnancy overweight (BMI: 25.9 kg/m2) were more likely to gain weight excessively in pregnancy (p < 0.001). Offspring of mothers who gained excess total gestational weight were significantly heavier at 1, 3, and 5 years compared to children whose mothers did not gain excess weight. Adipose tissue, measured by SFTs, abdominal ultrasound, and MRI, did not differ between the three groups of children.
Results from linear regression analyses that explored relationships between total GWG and offspring body composition are seen in Table 2. Apart from associations at birth and 1 year, no other significant relationships between child outcomes and total GWG were observed. We performed additional analyses to determine if associations between total GWG (kg) and offspring outcomes were modified by pre-pregnancy BMI by including their interaction terms in separate models and found no significant effect modification (data not shown).
Multiple linear regression models were fit to determine if early or mid-pregnancy weight gain was associated with offspring body composition. No significant associations were observed between GWG in early pregnancy and offspring clinical characteristics. However, mid-gestation weight gain was associated with offspring weight, BMI percentiles and SFTs at birth in the unadjusted models [β: 40.63 g (95% CI: 15.56; 65.69), p = 0.002; β: 1.53 %-ile (95% CI: 0.14; 2.91), p = 0.031, and β: 0.13 mm (95% CI: 0; 0.27), p = 0.049, respectively]. At 5 years, associations persisted in the unadjusted models between mid-pregnancy weight gain and child body weight [β: 149.61 g (95% CI: 11.71; 287.51)], as well as subcutaneous adipose tissue (SAT) volume by MRI [β: 20.11 cm3 (95% CI: 2.53; 37.69), p = 0.026]. Table 3 shows regression analyses of early and mid-pregnancy weight gain after confounder adjustments. Relationships between mid-gestation weight gain and offspring body weight and BMI percentiles at birth were observed [β: 44.79 g (95% CI: 24.40; 65.18), p < 0.001 and β: 1.72 %-ile (95% CI: 0.45; 2.99), p = 0.047, respectively]. At 5 years, mid-pregnancy weight gain was associated with offspring body weight, subcutaneous abdominal fat in the axial plane, and SAT-volume [β: 139.34 g (95% CI: –0.22; 278.90), p = 0.050; β: 1.42 mm2 (95% CI: 0.06; 2.78), p = 0.041; and β: 18.56 cm3 (95% CI: 1.30; 35.82), p = 0.036, respectively]. Similar results at 5 years were observed after excluding women with a BMI ≥ 25.0 kg/m2 [5 years: body weight β: 171.74 g (95% CI: 8.56; 334.92), p = 0.039; SAT-volume β: 20.69 mm2 (95% CI: 1.97; 39.41), p = 0.031].
Discussion
The purpose of this study was to explore associations between GWG and adiposity outcomes in early childhood. Almost half the women in our cohort gained more weight during pregnancy than recommended by the IOM, which is consistent with other studies,24 and those with a pre-pregnancy BMI ≥ 25.0 kg/m2 (n = 33) were more likely to gain in excess. Overall, mothers who gained excessive total gestational weight had children with a higher weight throughout infancy and early childhood as compared to children of mothers with non-excessive gain. We could not confirm that total GWG was independently associated with fat accretion in young children. However, we found strong evidence that mid-pregnancy weight gain predicted higher weight and larger volumes of subcutaneous abdominal fat at 5 years, albeit with small effect sizes.
Many studies have shown a link between GWG and the risk of offspring adiposity,4,12,24,25 although this relationship is not universally observed.26,27 A systematic review found that higher GWG increased the risk of overweight and obesity in childhood.3 However, the authors acknowledge that results should be interpreted with the caveat that considerable methodological differences exist across studies, particularly whether shared familial characteristics were controlled for in analyses. A population cohort study of over 130,000 families echos this caution by reporting that associations between maternal weight gain among normal-weight women and BMI in their 18-year-old offspring were attenuated after controlling for shared genetic and lifestyle characteristics.14 The IOM concludes that more research is needed to confirm definitively that excess GWG independently predicts obesity in children.2 It is also unclear whether weight gained in different gestational periods has varying effects on child outcomes, and studies that have explored these associations report inconsistent findings. Both the Generation R and Danish National Birth Cohorts observed that weight gain in early and mid-pregnancy was associated with childhood obesity outcomes.28,29 Conversely, some researchers have demonstrated associations between first-trimester GWG only and early adiposity,5,30,31 whereas others have observed relationships between excessive weight gain in both early and late pregnancy and increased obesity risk at 3 years old.32
Underlying mechanisms that explain why the timing of GWG could play a role in offspring fat development remain elusive. Excess weight gained early-on may confer obesity risk via a programming effect. Early pregnancy weight is largely composed of fat33 which results in a concomitant increase in nutrient availability for fetal growth. Alternatively, exposure to later GWG may directly contribute to the overall bodyweight of the fetus.2 Limited evidence from animal models found that excess GWG in later pregnancy led to dysregulation of offspring adipocytes resulting in fat accretion, even though total GWG was within the recommended range.34 Fetal adipose tissue formation is a multi-step process involving the conversion of precursor cells into adipocytes that takes place between the 14th and 24th weeks of gestation.35,36 It is possible that gaining excess gestational weight in the same time frame as fetal adipogenesis would enhance the differentiation of adipocyte precursor cells into adipocytes through epigenetic mechanisms,37 resulting in adipose tissue expansion in offspring. Our analysis showed that GWG in mid-pregnancy (from 15 to 32 weeks’ gestation) was associated with several adiposity outcomes at birth and 5 years. Findings were similar after we excluded women with overweight, which suggests that mid-pregnancy weight gain is associated with offspring clinical outcomes, irrespective of maternal pre-pregnancy BMI. The AVON study found similar results in the same window of pregnancy, but only among women who gained above the weekly IOM recommendations.6 Further studies are needed to confirm and extend our findings with larger and more diverse maternal/child cohorts.
Both pre-pregnancy BMI and excess GWG have been identified as modifiable risk factors of obesity in children.2 Many studies consider these two determinants as independent variables when investigating childhood adiposity outcomes. However, it is poorly understood how they interact to compound offspring obesity risk, and researchers have reported a combined effect of pre-pregnancy BMI and GWG on offspring growth.8,10,11,13,14,15,38,39,40,41 We found no evidence that pre-pregnancy BMI in our non-obese cohort amplified the effect of total GWG on offspring adiposity outcomes, which is in agreement with others.12,42 However, it is plausible that effects are more apparent in women who either begin their pregnancies with obesity, gain weight considerably above the IOM recommendations, or experience a combination of both.13,14,15,39,40 High pre-pregnancy BMI is a strong predictor of excessive weight gain in pregnancy.43 Notably, pregnant women who gain in excess have a higher likelihood of postpartum weight retention, thus beginning their next pregnancy with an even higher BMI.44 This promotes an intergenerational cycle of obesity and emphasizes the importance of addressing maternal determinants of offspring obesity risk as early as the pre-conception period. Thus, the compounded effect of pre-pregnancy BMI and GWG should also be taken into account when communicating the importance of avoiding excess weight gain among pregnant women, particularly for those who start their pregnancies with overweight/obesity.
A major strength of this study is our collection of body composition measurements using both indirect and direct methods that enabled us to assess growth and fat patterning in children over time. To our knowledge, the INFAT study is the first to track adipose tissue accretion via abdominal ultrasound in infancy and early childhood. This method has been validated as a reliable and reproducible tool for measuring adipose tissue expansion and distribution in young children.22,23
There are several limitations to consider. As with all cohort studies, there may be other confounding variables we did not adjust for that could influence outcomes. We considered the length of gestation as a confounder due to a significantly prolonged gestational period in the intervention group. This has also been documented in other studies with pregnant women who took n-3 LCPUFA supplements.45 We also adjusted for breastfeeding status, as breastfeeding is a putative determinant of clinical outcomes in children.46 We acknowledge that other maternal factors, such as smoking, are associated with childhood obesity.47 However, only five women (2.7%) in our cohort smoked in pregnancy; therefore, we chose not to include smoking as a confounder. Pre-pregnancy weight was self-reported, which may lead to under-reporting and could falsely inflate GWG values. We chose the early (<15 weeks) and mid-pregnancy (15–32 weeks) weight gain cut-off points because these were the time points for which we had maternal weight data. However, we cannot exclude the possibility that examining weight gain measurements from other time windows could lead to different results. For example, some evidence has shown that mothers who gain in excess during the first half of pregnancy (<20 weeks) give birth to heavier babies with more body fat.48,49 Further research with larger cohorts is warranted to explore the critical window in which maternal weight gain exerts the greatest influence on offspring obesity risk.
Our cohort consisted of mostly German, relatively well-educated women, with 69% reporting that they attended greater than 12 years of schooling. Hence, results may not be generalizable, and further studies in other socio-demographic, ethnic, and racial groups are needed to confirm findings. INFAT was designed as a proof-of-concept trial18,19 and women with obesity were excluded from the original study. Our cohort had a total of 33 women with a BMI ≥ 25.0 kg/m2 and results should be interpreted with caution. Additional studies with larger cohorts of pregnant women in higher BMI categories should be conducted to confirm our preliminary observations. Moreover, the power calculation was based on the primary study outcome (SFT measurements in the first year of life) and exploratory outcomes were not taken into account. Our results are also limited due to a small study population and considerable drop-out at the 5-year follow-up, although no significant differences in maternal characteristics were observed between our cohort and those lost to follow-up.
In conclusion, this study demonstrated that mid-gestation weight gain was associated with several adiposity outcomes at 5 years old. Our findings add to the body of literature suggesting that the timing of GWG affects offspring growth and fat development. These results highlight the importance of long-term follow-up of maternal-child cohorts to improve understanding of the effects of GWG, including the pattern of weight gain in pregnancy, on childhood obesity risk.
References
Oken, E. & Gillman, M. W. Fetal origins of obesity. Obes. Res. 11, 496–506 (2003).
Institute of Medicine. Weight Gain During Pregnancy: Reexamining the Guidelines (Washington, DC, 2009).
Lau, E. Y., Liu, J., Archer, E., McDonald, S. M. & Liu, J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J. Obes. 2014, 524939 (2014).
Mamun, A. A., Mannan, M. & Doi, S. A. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes. Rev. 15, 338–347 (2014).
Karachaliou, M. et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am. J. Obstet. Gynecol. 212, 502.e1–14 (2015).
Fraser, A. et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 121, 2557–2564 (2010).
Guo, L. et al. Gestational weight gain and overweight in children aged 3-6 years. J. Epidemiol. 25, 536–543 (2015).
Li, N. et al. Maternal prepregnancy body mass index and gestational weight gain on offspring overweight in early infancy. PLoS ONE 8, e77809 (2013).
Yu, Z. et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE 8, e61627 (2013).
Heerman, W. J., Bian, A., Shintani, A. & Barkin, S. L. Interaction between maternal prepregnancy body mass index and gestational weight gain shapes infant growth. Acad. Pediatr. 14, 463–470 (2014).
Nohr, E. A. et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am. J. Clin. Nutr. 87, 1750–1759 (2008).
Widen, E. M. et al. Gestational weight gain and obesity, adiposity and body size in African-American and Dominican children in the Bronx and Northern Manhattan. Matern. Child. Nutr. 12, 918–928 (2016).
Kaar, J. L. et al. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J. Pediatr. 165, 509–515 (2014).
Lawlor, D. A., Lichtenstein, P., Fraser, A. & Långström, N. Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am. J. Clin. Nutr. 94, 142–148 (2011).
Jin, W.-Y. et al. Independent and combined effects of maternal prepregnancy body mass index and gestational weight gain on offspring growth at 0–3 years of age. BioMed. Res. Int. 2016, 4720785 (2016).
Vanderwall, C., Eickhoff, J., Randall Clark, R. & Carrel, A. L. BMI z-score in obese children is a poor predictor of adiposity changes over time. BMC Pediatr. 18, 187 (2018).
Hu, F. B. Obesity Epidemiology xiii, 498 (Oxford University Press, Oxford, New York, 2008).
Hauner, H. et al. The impact of nutritional fatty acids during pregnancy and lactation on early human adipose tissue development. Rationale and design of the INFAT study. Ann. Nutr. Metab. 54, 97–103 (2009).
Hauner, H. et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open-label randomized controlled trial. Am. J. Clin. Nutr. 95, 383–394 (2012).
Brei, C. et al. Reduction of the n-6: n-3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: follow-up results from a randomized controlled trial up to 5 y of age. Am. J. Clin. Nutr. 103, 1472–1481 (2016).
Kromeyer-Hauschild, K. et al. Percentiles of body mass index in children and adolescents evaluated from different regional German studies. Monatsschr. Kinderheilkd. 8, 807–819 (2001).
Brei, C. et al. Sonographic assessment of abdominal fat distribution during the first year of infancy. Pediatr. Res. 78, 342–350 (2015).
Brei, C., Much, D., Brunner, S., Stecher, L. & Hauner, H. Longitudinal sonographic assessment of abdominal fat distribution from 2 to 5 years of age. Pediatr. Res. 84, 677–683 (2018).
Goldstein, R. F. et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 317, 2207–2225 (2017).
Oken, E., Rifas-Shiman, S. L., Field, A. E., Frazier, A. L. & Gillman, M. W. Maternal gestational weight gain and offspring weight in adolescence. Obstet. Gynecol. 112, 999–1006 (2008).
Whitaker, R. C. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114, e29–e36 (2004).
Sharp, G. C. et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 44, 1288–1304 (2015).
Andersen, C. S., Gamborg, M., Sorensen, T. I. & Nohr, E. A. Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. Int. J. Pediatr. Obes. 6, e179–e186 (2011).
Gaillard, R. et al. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy Obesity (Silver Spring) 21, 1046–1055 (2013).
Margerison-Zilko, C. E. et al. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern. Child Health J. 16, 1215–1223 (2012).
Lu, W. et al. Association between trimester-specific gestational weight gain and childhood obesity at 5 years of age: results from Shanghai Obesity Cohort. BMC Pediatr. 19, 139 (2019).
Diesel, J. C. et al. Is gestational weight gain associated with offspring obesity at 36 months? Pediatr. Obes. 10, 305–310 (2015).
Clapp, J. F., Seaward, B. L., Sleamaker, R. H. & Hiser, J. Maternal physiologic adaptations to early human pregnancy. Am. J. Obstet. Gynecol. 159, 1456–1460 (1988).
Giblin, L. et al. Offspring subcutaneous adipose markers are sensitive to the timing of maternal gestational weight gain. Reprod. Biol. Endocrinol. 13, 16 (2015).
Poissonnet, C. M., Burdi, A. R. & Bookstein, F. L. Growth and development of human adipose tissue during early gestation. Early Hum. Dev. 8, 1–11 (1983).
Sarr, O., Yang, K. & Regnault, T. R. In utero programming of later adiposity: the role of fetal growth restriction. J. Pregnancy 2012, 134758 (2012).
Ambele, M. A., Dhanraj, P., Giles, R. & Pepper, M. S. Adipogenesis: a complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 21 https://doi.org/10.3390/ijms21124283 (2020).
Wrotniak, B. H., Shults, J., Butts, S. & Stettler, N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am. J. Clin. Nutr. 87, 1818–1824 (2008).
Hinkle, S. N. et al. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J. Nutr. 142, 1851–1858 (2012).
Voerman, E. et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 16, e1002744 (2019).
Hunt, K. J., Alanis, M. C., Johnson, E. R., Mayorga, M. E. & Korte, J. E. Maternal pre-pregnancy weight and gestational weight gain and their association with birthweight with a focus on racial differences. Matern. Child Health J. 17, 85–94 (2013).
Deierlein, A. L., Siega-Riz, A. M., Adair, L. S. & Herring, A. H. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J. Pediatr. 158, 221–226 (2011).
Samura, T. et al. Factors associated with excessive gestational weight gain: review of current literature. Glob. Adv. Health Med. 5, 87–93 (2016).
Nehring, I., Schmoll, S., Beyerlein, A., Hauner, H. & Kries, Rvon Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am. J. Clin. Nutr. 94, 1225–1231 (2011).
Olsen, S. F. et al. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet 2, 367–369 (1986).
Harder, T., Bergmann, R., Kallischnigg, G. & Plagemann, A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am. J. Epidemiol. 162, 397–403 (2005).
Ino, T. Maternal smoking during pregnancy and offspring obesity: meta-analysis. Pediatrics Int. 52, 94–99 (2010).
Catov, J. M., Abatemarco, D., Althouse, A., Davis, E. M. & Hubel, C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity (Silver Spring) 23, 1071–1078 (2015).
Davenport, M. H., Ruchat, S.-M., Giroux, I., Sopper, M. M. & Mottola, M. F. Timing of excessive pregnancy-related weight gain and offspring adiposity at birth. Obstet. Gynecol. 122, 255–261 (2013).
Acknowledgements
We would like to thank the INFAT parents and their children for participating in the study. This study was funded by the following grants, foundations, and research centers: Else Kröner-Fresenius Foundation, Bad Homburg, Germany; International Unilever Foundation, Hamburg, Germany; EU-funded EARNEST (Early Nutrition Programming Project) consortium (FOOD-CT-2005-007036), administratively facilitated by Frank Wiens; German Ministry of Education and Research via the Competence Network on Obesity (Kompetenznetz Adipositas, 01GI0842), Germany; Danone Research-Center for Specialized Nutrition, Friedrichsdorf, Germany.
Author information
Authors and Affiliations
Contributions
H.H. conceived the original study design. D.M.M. analyzed data. All authors were involved in writing and revising the paper and had final approval of the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written consent was obtained from the participants of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meyer, D.M., Stecher, L., Brei, C. et al. Mid-pregnancy weight gain is associated with offspring adiposity outcomes in early childhood. Pediatr Res 90, 390–396 (2021). https://doi.org/10.1038/s41390-020-01202-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01202-x
This article is cited by
-
Longitudinal associations of pre-pregnancy BMI and gestational weight gain with maternal urinary metabolites: an NYU CHES study
International Journal of Obesity (2022)