Abstract
Background
Despite increasing use of propofol in neonates, observations on cerebral effects are limited.
Aim
To investigate cerebral autoregulation (CAR) and activity after propofol for endotracheal intubation in preterm neonates.
Methods
Twenty-two neonates received propofol before intubation as part of a published dose-finding study. Mean arterial blood pressure (MABP), near-infrared spectroscopy-derived cerebral oxygenation (rScO2), and amplitude-integrated electroencephalography (aEEG) were analyzed until 180 min after propofol. CAR was expressed as transfer function (TF) gain, indicating % change in rScO2 per 1 mmHg change in MABP. Values exceeding mean TF gain + 2 standard deviations (SD) defined impaired CAR.
Results
After intubation with a median propofol dose of 1 (0.5–4.5) mg/kg, rScO2 remained stable during decreasing MABP. Mean (±SD) TF gain was 0.8 (±0.3)%/mmHg. Impaired CAR was identified in 1 and 5 patient(s) during drug-related hypotension and normal to raised MABP, respectively. Suppressed aEEG was observed up to 60 min after propofol.
Conclusions
Drug-related hypotension and decreased cerebral activity after intubation with low propofol doses in preterm neonates were observed, without evidence of cerebral ischemic hypoxia. CAR remained intact during drug-related hypotension in 95.5% of patients. Cerebral monitoring including CAR clarifies the cerebral impact of MABP fluctuations.
Similar content being viewed by others
INTRODUCTION
Propofol, as intravenous (IV) bolus, is used as premedication for (semi-)elective intubation in neonates.1 It results in quick and effective sedation compared to morphine, atropine, and suxamethonium.2 These properties make propofol suitable for the INSURE (intubation, intratracheal administration of surfactant, and immediate extubation) procedure because presence or fast return of spontaneous breathing is mandatory for early extubation. Recently, the effective propofol dose for 50% of patients (ED50) in preterms for (semi-)elective intubation was provided.3 The reported ED50 is lower in this age group compared to infants aged 1–6 months.4 Arterial hypotension after propofol has been described, and is considered as drug-related hypotension. If mean arterial blood pressure (MABP) falls below postmenstrual age (PMA) but with evidence of good tissue perfusion, treatment is not always initiated, equivalent to a transitional low blood pressure phenomenon in preterm infants.5,6,7 Although MABP may not be the optimal parameter to assess cerebral circulation and end-organ perfusion,8 this finding prevents clinicians from the use of propofol in (preterm) neonates.9
Cerebral autoregulation (CAR) is the capacity to maintain cerebral blood flow (CBF) in a wide range of cerebral perfusion pressures (CPP).10 CPP is determined by MABP and intracranial pressure. If intracranial pressure is stable, MABP can be used as a surrogate for CPP.11 However, CAR mechanisms may fail. Subsequently, decreased or increased CBF caused by fluctuations in MABP may jeopardize the neonatal brain.12 Near-infrared spectroscopy (NIRS)-derived regional cerebral oxygen saturation (rScO2) is a continuously measured, bedside surrogate of CBF if arterial oxygen saturation (SaO2) measurements and cerebral metabolism are stable. CAR can be calculated using different mathematical models to describe the similarities between the dynamically changing rScO2 and MABP signals.13 This is especially of interest during fluctuations in MABP (i.e., drug-related hypotension).14
Multi-modal monitoring of physiological measurements with amplitude-integrated electroencephalography (aEEG) and cerebral fractional tissue oxygen extraction (cFTOE) can elucidate the effect of sedative drugs on cerebral activity and cerebral metabolism.15,16 cFTOE, calculated as ((SaO2 − rScO2)/SaO2), hereby reflects the balance between oxygen delivery determined by CBF and SaO2, and oxygen consumption reflecting the cerebral metabolism.17
Despite increasing use of propofol in neonatal care, data on CAR and cerebral activity are absent. The aim of this study is to investigate CAR and cerebral activity after premedication with propofol for endotracheal intubation in preterm neonates.
METHODS
Clinical characteristics and outcome
Neonates of a published propofol dose-finding study (NEOPROP study) were considered for inclusion.3 The NEOPROP study was performed in the neonatal intensive care unit of the University Hospitals Leuven, Belgium. Hemodynamically stable neonates needing pre-intubation sedation were included after informed written consent of the parent(s). The study was registered at ClinicalTrials.gov (NCT01621373) with EudraCT 2012-002648-26 and approved by the institutional review board of the University Hospitals Leuven.3 Clinical characteristics were extracted from the medical files. Propofol (0.5–4.5 mg/kg) bolus was administered according to study protocol. Time of first propofol administration (T0) and time to intubation and extubation (expressed as minutes after propofol administration) were noted. For further details concerning propofol indication, the dose-finding approach and pharmacodynamic assessments we refer to the initial publication.3
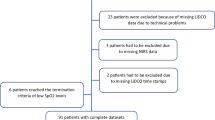
Inclusion criteria for the current analysis were neonates with gestational age (GA) <37 weeks, receiving the INSURE procedure with simultaneous collection of invasive MABP, rScO2, and amplitude-integrated electroencephalography (aEEG) with impedance <10 kOhm. Patients receiving a sedative agent up to 180 min after propofol were excluded. Three groups according to postmenstrual age (PMA) were defined (group 1: <28, group 2: 28 to <32, group 3: 32 to <37 weeks). Successful INSURE procedure was defined as successful extubation within 1 h. Cranial ultrasound data were collected. Intraventricular hemorrhage (IVH) was classified according to Papile.18 Periventricular echodensities (PVE) were diagnosed if inhomogeneous, hyperechogenic lesion(s) in the periventricular white matter persisted beyond postnatal age (PNA) of 14 days.19
Vital signs and cerebral oxygenation
Vital signs [heart rate (HR)(beats per minute), invasive MABP (mmHg), and SaO2 (%)] were measured using IntelliVue MP70 (Philips, The Netherlands). rScO2 (%), measured by INVOS 5100 and the neonatal OxyAlert NIRSensor (Covidien, Mansfield, Massachusetts) was used to estimate changes in regional cerebral oxygenation. Data were recorded simultaneously and continuously with a sampling rate of 1 Hertz using Rugloop (Demed, Temse, Belgium). Data were analyzed up to 180 min after propofol using MATLAB 2013a (The Mathworks, Natick, Massachusetts) to provide trend lines. Median values of the vital signs were obtained in 2 min-epochs from 2 min before propofol (i.e., baseline) up to 20 min afterwards and from 10 min-epochs at 30, 60, 120, and 180 min after propofol and expressed as ratio versus baseline. Drug-related hypotension was defined as MABP below PMA after propofol administration. The corrected MABP (cMABP = MABP − PMA) fluctuations below 0 mmHg were quantified by the area under the curve (AUC) of the cMABP versus time curve when cMABP was negative, using trapezoidal numerical integration.3 Consequently, AUC cMABP indicates the extent and duration, and thus severity, of drug-related hypotension in each patient.
Cerebral autoregulation
CAR was calculated using coherence (COH) and TF (transfer function) gain between dynamically changing rScO2 and MABP. COH, defined as correlation in the frequency domain between MABP and rScO2, exceeding the threshold for significance indicates that CAR is pressure passive. TF gain, defining the transfer magnitude, quantifies the damping effect between the input (MABP) and the output (rScO2) signal and is expressed as % change in rScO2 per 1 mmHg change in MABP in coherent epochs.20,21 Artifact removal in the acquired data was performed by visual inspection of the signals. Segments with large variation caused by movement artifacts or suboptimal signal quality, were removed from further analysis. SaO2 has a major influence on NIRS derived cerebral oxygenation. This means that a decrease in SaO2 can result in a hypoxic (low oxygen content) cerebral desaturation but not necessarily an ischemic (low blood flow) cerebral desaturation. Therefore, rScO2 was corrected for changes in SaO2 using oblique subspace projections.22 This mathematical model decouples the linked dynamics between different underlying subsystems (SaO2 and CBF) in order to decompose the observed output (rScO2) in terms of the partial contributions of each input variable. The contribution of SaO2 in the rScO2 signal is hereby eliminated, which makes the residual component a more correct surrogate for CBF, suitable for the assessment of CAR. To study CAR during the INSURE procedure, consecutive MABP and rScO2 epochs of 20 min with overlap of 19 min were used. For each epoch, COH and TF gain, using frequency analysis in overlapping sub-windows of 5 min, were calculated in the 0.003–0.02 Hz frequency band until 180 min after propofol and analyzed using MATLAB. To define the COH threshold to identify pressure-passive epochs, Monte Carlo simulations were performed with the same length as the processed segments. During 0–60, 60–120, and 120–180 min after propofol, TF gain was plotted against the mean MABP in the respective pressure-passive epoch and presented across MABP bins of 5 mmHg per patient and per group. Median TF gain was calculated for each patient over 180 min. The mean of each patients median TF gain + 2 standard deviations (SD) was used as threshold for the median TF gain across each MABP bin to define episodes of impaired CAR.
Cerebral activity
The qualitative aEEG (Olympic CFM 6000, Natus Medical Incorporated, Seattle) analysis, using biparietal C3–C4 electrodes (Neuroline Cup, Ambu, Ballerup, Denmark), was performed separately by LT and AD in a 15 min-epoch before propofol (i.e., baseline) and 60 min-epochs at 0–60, 60–120, and 120–180 min after propofol using the Burdjalov score expressing background pattern.23 Epochs with inter-rater disagreement were re-evaluated and a final score was assigned by consensus.
Statistical analysis
Clinical characteristics are reported as median (range) or incidence. Data analysis and Cohen’s κ was performed using SPSS version 24 (IBM Corp, Armonk, New York) and SAS 9.4 for Windows (SAS Institute, Inc., Cary, North Carolina). Evolution of vital signs over time-epochs compared between groups was determined using a multivariate linear model for longitudinal measures with a compound symmetric covariance matrix on log-scale and expressed as ratio versus baseline. Bonferroni–Holm adjustments for multiple testing are reported. A p-value <0.05 was considered statistically significant.
RESULTS
Twenty-two of 50 neonates of the NEOPROP study were included in the current analysis.3 Clinical characteristics are presented in Table 1. Severe abnormalities on cranial ultrasound (IVH grade 3/4, cystic periventricular leukomalacia, ventriculomegaly) were not observed in the study population.
Vital signs and cerebral oxygenation
After propofol administration, decrease in MABP (Fig. 1a) was observed in all groups, most pronounced after 12–14 min in group 3 [mean (CI) ratio versus baseline for MABP: 0.77 (0.66;0.90)] with return to baseline values 120 min after propofol. The MABP evolution over time did not differ significantly between groups (F-value 0.57; p = 0.954). Median (range) AUC cMABP was 2(0-9659) and drug-related hypotension (AUC cMABP > 0) was diagnosed in 11/22 patients. No treatment was started by the treating physician. AUC cMABP was equal to 0 in 11/22 patients with cMABP never below 0 mmHg.
The trend of MABP (a), SaO2 (b), rScO2 (c), and cFTOE (d) from baseline (i.e., 0–2 min before propofol administration) over predefined time-epochs is plotted as least-squares means and their 95% confidence interval for the different PMA groups. cFTOE cerebral fractional tissue oxygen extraction, MABP mean arterial blood pressure, PMA postmenstrual age, rScO2 regional cerebral oxygen saturation, SaO2 peripheral oxygen saturation, w weeks. *p < 0.001 for PMA <28 versus PMA 28 to <32 weeks
Significantly lower SaO2 (Fig. 1b) and rScO2 (Fig. 1c) values were observed around the INSURE procedure (i.e., 4–6 min after propofol) in group 1 versus 2 [mean (CI) ratio versus baseline for SaO2: 0.80 (0.74;0.87) versus 1.05 (0.99;1.11), p ≤ 0.0001 and rScO2: 0.77 (0.68;0.89) versus 1.14 (1.04;1.23) p = 0.0003]. After the intubation procedure, rScO2 remained stable with a trend to increase above baseline values up to 180 min after propofol. Increased cFTOE (Fig. 1d) is observed in group 1 during the INSURE procedure, in conjunction with the previously described peripheral and cerebral desaturation [mean (CI) ratio versus baseline for cFTOE: 1.06 (0.67;1.68)]. Subsequently, cFTOE decreased below baseline during the first 30 min in all groups. The evolution of cFTOE over time was not significantly different between the groups (F-value 0.39; p = 0.997). The results of the model were influenced by 1 outlier for rScO2 in group 1 and 2 outliers for SaO2 in group 1 and 3. After exclusion of these data points, significant differences in rScO2 over time between groups disappeared. We decided to report the original results to reflect the clinical reality during the INSURE procedure.
Cerebral autoregulation
For each patient, 164 epochs were analyzed. Using Monte Carlo simulations, a threshold of 0.3 for COH between rScO2 and MABP was identified. Median (range) COH was 0.3 (0.1–0.6), 0.3 (0.1–0.6) and 0.3 (0.1–0.7) in group 1, 2, and 3, respectively. A median (range) of 86(54–119) epochs per patient were defined as pressure passive (COH > 0.3). Median (range) value of TF gain was 0.9 (0.8–1.5), 0.8 (0.4–1.3), and 0.9 (0.4–1.3) in group 1, 2, and 3, respectively. Mean (±SD) of the median TF gain in the total cohort was 0.8 (±0.3)%/mmHg. A threshold for median TF gain of 1.4%/mmHg was defined to indicate episodes of impaired CAR. Median TF gain remained below 1.4%/mmHg across each MABP bin after propofol in 16/22 patients (Fig. 2). Highest values of median TF gain were observed in group 1. After review of the individual plots, 6 patients with median TF gain >1.4%/mmHg across specific MABP bins during 0–60, 60–120, and/or 120–180 min after propofol were identified. In 1 case, this occurred in the first 60 min after propofol during a period of drug-related hypotension (Fig. 3). In 5 cases, median TF gain >1.4%/mmHg was observed across MABP bins with normal to raised MABP. Thus, intact CAR during drug-related hypotension was found in 21/22 (95.5%) of patients. No association was found between presence of impaired CAR and cranial ultrasound abnormalities.
For the different PMA groups, boxplots of TF gain values (Y-axis, %/mmHg) are plotted against the mean MABP in the respective pressure-passive epoch. These epoch’s mean MABP values are presented across MABP bins of 5 mmHg (X-axis). Graphs are provided for 0–60, 60–120, and 120–180 min after propofol administration. The mean (0.8%/mmHg) and mean + 2 SD (1.4%/mmHg) of the cohort median TF gain are indicated as a solid and dashed horizontal line, respectively. MABP mean arterial blood pressure, min minutes, PMA postmenstrual age, rScO2 regional cerebral oxygen saturation, SD standard deviation, TF transfer function
In a patient with propofol-related hypotension, bars (median with interquartile range) of TF gain values (Y-axis, %/mmHg) are plotted against the mean MABP in the respective pressure-passive epoch. These epoch’s mean MABP values are presented across MABP bins of 5 mmHg (X-axis). Graphs are provided for 0–60, 60–120, and 120–180 min after propofol administration (left). TF gain of 1.4%/mmHg as the threshold for impaired CAR is indicated as a dashed horizontal line. Parameter trend lines until 180 min after propofol administration are displayed: MABP (middle above), SaO2 (right above), and rScO2 (middle below). CAR cerebral autoregulation, MABP mean arterial blood pressure, rScO2 regional cerebral oxygen saturation, SaO2 arterial oxygen saturation, TF transfer function
Cerebral activity
The maturational difference of the baseline Burdjalov score was observed among the three age groups (Fig. 4). For the qualitative analysis of 85 aEEG epochs (three missing baseline epochs), a Cohen’s κ of 0.576 (p < 0.0005) was reached, indicating moderate inter-rater agreement. After propofol, the change over time in Burdjalov scores did not differ between groups. However, the baseline score was significantly different from the score at 0–60 min for each group [mean (CI) Burdjalov score at baseline versus 0–60 min for group 1: −3.88 (−6.27;−1.49) p = 0.03, group 2: −4.23 (−5.64;−2.81) p < 0.0001 and group 3: −5.88 (−8.61;−3.15) p = 0.001] (Fig. 4). This difference from baseline disappeared at 60–120 min after propofol and indicates suppressed cerebral activity 0–60 min after propofol administration.
DISCUSSION
Intact cerebral autoregulation during suppressed cerebral activity
This study describes assessment of CAR during propofol-related decrease in MABP in preterm neonates receiving 0.5–4.5 mg/kg IV propofol bolus for the INSURE procedure. We describe intact CAR during episodes of drug-related hypotension in 95.5% of patients. A COH threshold of 0.3 and median TF gain across MABP bins exceeding 1.4%/mmHg were used to identify episodes of impaired CAR. This TF gain threshold was defined based on the mean + 2 SD of the median TF gain calculated for each neonate. Therefore, above this threshold, pressure-passive CBF with a magnitude exceeding the mean + 2 SD of the cohort is observed. Normal TF gain values for this age group are currently not available. The mathematical models COH and TF gain to assess CAR are validated in piglets.24 Moreover, in a group of 24 preterm neonates, a significant association was found between higher mean TF gain in pressure-passive epochs with COH ≥ 0.5 and mortality.25 Importantly, comparison of absolute TF gain values is impossible because different mathematical methods were used.25
In this study, suppressed cerebral activity is observed during the first 60 min after propofol administration for endotracheal intubation. This finding demonstrates the prolonged effect of propofol on electrical brain activity in preterms, despite recovery of the clinical condition and the achievement of early extubation in most cases. This study demonstrates that the propofol-induced decrease in blood pressure and cerebral activity up to 60 min results in stable rScO2 values with low values of cFTOE (low oxygen extraction), suggesting decreased metabolism without cerebral ischemic hypoxia. This reflects adequate CBF despite low MABP with an adequate balance between oxygen delivery and extraction and is in line with the reports of Vanderhaegen et al.6 and Garner et al.8 The observation of intact CAR, even during drug-related hypotension in 95.5% of patients, strengthens this hypothesis. If the lower threshold of autoregulation is reached, CBF would be pressure passive and a decrease in systemic MABP would result in lower cerebral oxygen delivery with subsequent higher oxygen extraction and an increase in TF gain.
This can be considered as an argument in favor of propofol, administered as low bolus doses, used as premedication for (semi-)elective intubation in preterm neonates. As recommended by Smits et al., and until validated dosing regimens appear, published propofol ED50 doses (effective dose in 50% of patients) can be administered at induction with subsequent up-titration of the dose based on clinical need.3 Whether drug-related hypotension is also associated with intact CAR in preterm neonates with other clinical conditions and/or other sedative drugs needs further study.
Impaired cerebral autoregulation
Median TF gain >1.4%/mmHg was observed across MABP bins of 6 patients. No counter phase in MABP and rScO2 leading to falsely elevated TF gain, as described by Eriksen et al.,26 was observed during these episodes of impaired CAR. In 1 patient, TF gain exceeding the threshold was observed during drug-related hypotension (Fig. 3). This preterm of 27 weeks PMA with intra-uterine growth restriction (IUGR) and brain sparing underwent a successful INSURE procedure. Cranial ultrasound showed PVE < 14 days. We hypothesize that the increased TF gain and thus impaired CAR can be explained by cerebral vasodilatation due to IUGR and brain sparing.27 Consequently, this case illustrates the theory postulated by Scherjon28 in 1994: ‘Intra-uterine stress might, in analogy with the acceleration of lung maturation, induce a physiological alteration leading to a different setting of the autoregulation of the cerebral circulation’. Alternatively, maternal treatment with magnesium sulfate and nifedipine might have influenced CAR mechanisms.29,30
In 5 patients, episodes of impaired CAR were observed during normal to raised MABP. We hypothesize this finding might reflect the upper inflection point of the CAR curve and is best visible around 39 mmHg in group 1 at 0–60 and 60–120 min after propofol administration (Fig. 2). In the present study, highest values of TF gain were observed in group 1 (PMA < 28 weeks). This is in line with the findings of Vesoulis.31 Whether this indeed indicates impaired CAR in 4/5 patients or reflects age-appropriate normal values of pressure-passive CAR needs to be explored in a larger cohort. The long-term consequence of this finding is at present unclear. Although episodes of impaired CAR were observed in 6 patients, no severe lesions on cranial ultrasound were diagnosed.
Study limitations and future perspectives
First of all, the question arises whether during changes in cerebral activity or metabolic rate, rScO2 is still a valid surrogate of CBF. We hypothesize CAR assessment can still be performed, but the question is not if CBF remains stable but rather adequate (not too high, not too low) to fulfill the brain metabolic needs and to avoid cerebral ischemic hypoxia. With the observation of decreasing values of cFTOE, CBF is, in our opinion, adequate and at least not too low despite the decrease in MABP. Therefore, a CAR mechanism must be present and we consider TF gain assessment as a valid mathematical model to explore. By plotting TF gain across MABP bins, it is possible to determine the blood pressure ranges involved during impaired CAR.
Secondly, the definition of impaired CAR was based on a cohort-based threshold. Although no major complications were documented, (ab)normal TF gain values need further identification and subsequent validation in larger cohorts. Third, we only included hemodynamically stable patients in need of an INSURE procedure. Extrapolation to other, critically ill neonates and/or other propofol indications is not recommended. Fourth, TF gain values were calculated from measurements starting 2 min before propofol administration (i.e., baseline recording) resulting in available TF gain values starting from 18 min after propofol. Consequently, individual baseline measurements covering a longer period before propofol are not available. Fifth, the additional impact of intubation and surfactant administration on the cerebral observations has to be taken into account. Concerning CBF, different studies have demonstrated no changes in CBF and cerebral oxygenation during surfactant administration.32,33 Suppression of cortical activity after surfactant treatment with or without premedication and intubation is described, ranging from 10 to 30 min after surfactant, and up to 24 h if premedication with morphine was used.34,35,36 In the current study, it is impossible to disentangle the impact of intubation or surfactant from propofol on brain activity.
Propofol is widely used for short procedural sedation and induction of anesthesia in (preterm) neonates, although overall safety data are limited.3 At present, there are no well established guidelines for blood pressure management in neonates. There is no evidence that neonates with numerically low MABP but good perfusion have poorer outcomes, nor that treatment improves outcome.37 Cerebral monitoring (rScO2 and aEEG) with assessment of TF gain gives insight into the cerebral impact of fluctuations in MABP. If applied as a real-time, bedside tool, treatment decisions guided by the patients CAR status might be possible. Furthermore, this propofol model with CAR capacity, cerebral activity, and short-term neurological outcome can be used to assess effects of other drugs in this vulnerable patient population. Whether the assessment of bedside CAR status can be used to identify patients at risk or individualize treatment to improve short-term and long-term outcome needs further study.
In conclusion, drug-related hypotension and decreased cerebral activity after low propofol doses for endotracheal intubation in preterm neonates were observed, without evidence of cerebral ischemic hypoxia. CAR mechanisms remained intact during this drug-related hypotension in 95.5% of patients. Cerebral monitoring with CAR measurement gives insight into the cerebral impact of MABP fluctuations.
References
Barrington, K. Premedication for endotracheal intubation in the newborn infant. Paediatr. Child Health 16, 159–171 (2011).
Ghanta, S. et al. Propofol compared with the morphine, atropine, and suxamethonium regimen as induction agents for neonatal endotracheal intubation: a randomized, controlled trial. Pediatrics 119, e1248–e1255 (2007).
Smits, A. et al. Propofol dose-finding to reach optimal effect for (semi-)elective intubation in neonates. J. Pediatr. 179, 54–60 e59 (2016).
Westrin, P. The induction dose of propofol in infants 1–6 months of age and in children 10-16 years of age. Anesthesiology 74, 455–458 (1991).
Simons, S. H. et al. Clinical evaluation of propofol as sedative for endotracheal intubation in neonates. Acta Paediatr. 102, e487–e492 (2013).
Vanderhaegen, J. et al. Cerebral and systemic hemodynamic effects of intravenous bolus administration of propofol in neonates. Neonatology 98, 57–63 (2010).
Dempsey, E. M., Al Hazzani, F. & Barrington, K. J. Permissive hypotension in the extremely low birthweight infant with signs of good perfusion. Arch. Dis. Child Fetal Neonatal Ed. 94, F241–F244 (2009).
Garner, R. S. & Burchfield, D. J. Treatment of presumed hypotension in very low birthweight neonates: effects on regional cerebral oxygenation. Arch. Dis. Child Fetal Neonatal Ed. 98, F117–F121 (2013).
Welzing, L. et al. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr. Anaesth. 20, 605–611 (2010).
Lassen, N. A. Cerebral blood flow and oxygen consumption in man. Physiol. Rev. 39, 183–238 (1959).
Panerai, R. B. et al. Assessment of dynamic cerebral autoregulation based on spontaneous fluctuations in arterial blood pressure and intracranial pressure. Physiol. Meas. 23, 59–72 (2002).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 (2009).
Thewissen, L. et al. Measuring near-infrared spectroscopy derived cerebral autoregulation in neonates: from research tool toward bedside multimodal monitoring. Front. Pediatr. 6, 117 (2018).
Wong, F. Y. et al. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS ONE 7, e43165 (2012).
Hutt, A. The anesthetic propofol shifts the frequency of maximum spectral power in EEG during general anesthesia: analytical insights from a linear model. Front. Comput. Neurosci. 7, 2 (2013).
Akeju, O. et al. Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 121, 990–998 (2014).
Naulaers, G. et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology 92, 120–126 (2007).
Papile, L. A. et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
de Vries, L. S., Eken, P. & Dubowitz, L. M. The spectrum of leukomalacia using cranial ultrasound. Behav. Brain Res. 49, 1–6 (1992).
Caicedo, A. et al. Detection of cerebral autoregulation by near-infrared spectroscopy in neonates: performance analysis of measurement methods. J. Biomed. Opt. 17, 117003 (2012).
Claassen, J. A. et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow. Metab. 36, 665–680 (2016).
Caicedo, A. et al. Decomposition of near-infrared spectroscopy signals using oblique subspace projections: applications in brain hemodynamic monitoring. Front. Physiol. 7, 515 (2016).
Burdjalov, V. F., Baumgart, S. & Spitzer, A. R. Cerebral function monitoring: a new scoring system for the evaluation of brain maturation in neonates. Pediatrics 112, 855–861 (2003).
Hahn, G. H. et al. Applicability of near-infrared spectroscopy to measure cerebral autoregulation noninvasively in neonates: a validation study in piglets. Pediatr. Res. 70, 166–170 (2011).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Eriksen, V. R., Hahn, G. H. & Greisen, G. Cerebral autoregulation in the preterm newborn using near-infrared spectroscopy: a comparison of time-domain and frequency-domain analyses. J. Biomed. Opt. 20, 037009 (2015).
Cohen, E. et al. Growth restriction and gender influence cerebral oxygenation in preterm neonates. Arch. Dis. Child Fetal Neonatal Ed. 101, F156–F161 (2016).
Scherjon, S. A. et al. Effect of fetal brainsparing on the early neonatal cerebral circulation. Arch. Dis. Child Fetal Neonatal Ed. 71, F11–F15 (1994).
Caicedo, A. et al. Effect of maternal use of labetalol on the cerebral autoregulation in premature infants. Adv. Exp. Med Biol. 789, 105–111 (2013).
Richter, A. E. et al. The effect of maternal antihypertensive drugs on the cerebral, renal and splanchnic tissue oxygen extraction of preterm neonates. Neonatology 110, 163–171 (2016).
Vesoulis, Z. A. et al. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr. Res. 79, 453–459 (2016).
Skov, L., Bell, A. & Greisen, G. Surfactant administration and the cerebral circulation. Neonatology 61, 31–36 (1992).
Skov, L. et al. Acute changes in cerebral oxygenation and cerebral blood volume in preterm infants during surfactant treatment. Neuropediatrics 23, 126–130 (1992).
van den Berg, E. et al. Effect of the “InSurE” procedure on cerebral oxygenation and electrical brain activity of the preterm infant. Arch. Dis. Child Fetal Neonatal Ed. 95, F53–F58 (2010).
Shangle, C. E. et al. Effects of endotracheal intubation and surfactant on a 3-channel neonatal electroencephalogram. J. Pediatr. 161, 252–257 (2012).
Smits, A., van den Anker, J. N. & Allegaert, K. Clinical pharmacology of analgosedatives in neonates: ways to improve their safe and effective use. J. Pharm. Pharmacol. 69, 350–360 (2017).
Barrington, K. J. Low blood pressure in extremely preterm infants: does treatment affect outcome? Arch. Dis. Child Fetal Neonatal Ed. 96, F316–F317 (2011).
Acknowledgements
We thank Steffen Fieuws (Leuven Biostatistics and Statistical Bioinformatics Center, L-Biostat, KU Leuven) for statistical advice, and all patients, parents and nurses participating in the study. L.T., A.C., and G.N. are funded by EU: European Union’s Seventh Framework Program (FP7/2007-2013) The HIP Trial: #260777. A.C. is a postdoctoral fellow of the Fund for Scientific Research, Flanders (Belgium) (FWO Vlaanderen) and received funding from imec funds 2017. The research activities of A.S. are supported by the Clinical Research and Education Council of the University Hospitals Leuven.
Author information
Authors and Affiliations
Contributions
Dr. L.T. conceptualized and designed the study and study methodology, collected the study data, carried out the analyses, wrote the first draft of the manuscript, and revised the manuscript. A.C. conceptualized and designed the study and study methodology, carried out the analyses. and critically reviewed the manuscript. Dr. A.D. contributed to the methodology of the study, carried out analyses, and critically reviewed the manuscript. S.V.H. contributed to the methodology of the study and critically reviewed the manuscript. Dr. G.N. and Dr. K.A. conceptualized and designed the study and study methodology, and critically reviewed the manuscript. Dr. A.S. conceptualized and designed the study and study methodology, collected the study data, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thewissen, L., Caicedo, A., Dereymaeker, A. et al. Cerebral autoregulation and activity after propofol for endotracheal intubation in preterm neonates. Pediatr Res 84, 719–725 (2018). https://doi.org/10.1038/s41390-018-0160-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0160-3
This article is cited by
-
Near-infrared spectroscopy monitoring of neonatal cerebrovascular reactivity: where are we now?
Pediatric Research (2023)
-
Enhanced INSURE (ENSURE): an updated and standardised reference for surfactant administration
European Journal of Pediatrics (2022)
-
Premedication with ketamine or propofol for less invasive surfactant administration (LISA): observational study in the delivery room
European Journal of Pediatrics (2021)