Abstract
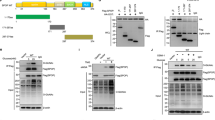
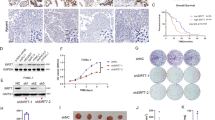
O-linked-β-N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation) and ubiquitination are critical posttranslational modifications that regulate tumor development and progression. The continuous progression of the cell cycle is the fundamental cause of tumor proliferation. S-phase kinase-associated protein 2 (SKP2), an important E3 ubiquitin ligase, assumes a pivotal function in the regulation of the cell cycle. However, it is still unclear whether SKP2 is an effector of O-GlcNAcylation that affects tumor progression. In this study, we found that SKP2 interacted with O-GlcNAc transferase (OGT) and was highly O-GlcNAcylated in hepatocellular carcinoma (HCC). Mechanistically, the O-GlcNAcylation at Ser34 stabilized SKP2 by reducing its ubiquitination and degradation mediated by APC-CDH1. Moreover, the O-GlcNAcylation of SKP2 enhanced its binding ability with SKP1, thereby enhancing its ubiquitin ligase function. Consequently, SKP2 facilitated the transition from the G1-S phase of the cell cycle by promoting the ubiquitin degradation of cell cycle-dependent kinase inhibitors p27 and p21. Additionally, targeting the O-GlcNAcylation of SKP2 significantly suppressed the proliferation of HCC. Altogether, our findings reveal that O-GlcNAcylation, a novel posttranslational modification of SKP2, plays a crucial role in promoting HCC proliferation, and targeting the O-GlcNAcylation of SKP2 may become a new therapeutic strategy to impede the progression of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The original mass spectrometry analysis data of SKP2 O-GlcNAcylation was deposited in the integrated proteome resource (iProX, PXD048234). All relevant data are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71:209–49.
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17:139–52.
Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17:666–72.
Hershko A, Ciechanover A, Varshavsky A. Basic medical research award. The ubiquitin system. Nat Med. 2000;6:1073–81.
Berndsen CE, Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol. 2014;21:301–7.
Zheng N, Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu Rev Biochem. 2017;86:129–57.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53.
Sutterlüty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Biol. 1999;1:207–14.
Latres E, Chiarle R, Schulman BA, Pavletich NP, Pellicer A, Inghirami G, et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA. 2001;98:2515–20.
Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci USA. 1998;95:11324–9.
Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–7.
Chan C-H, Lee S-W, Wang J, Lin H-K. Regulation of Skp2 expression and activity and its role in cancer progression. Sci World J. 2010;10:1001–15.
Ben-Izhak O, Lahav-Baratz S, Meretyk S, Ben-Eliezer S, Sabo E, Dirnfeld M, et al. SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. J Urol. 2003;170:241–5.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Chang YH, Weng CL, Lin KI. O-GlcNAcylation and its role in the immune system. J Biomed Sci. 2020;27:57.
Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22.
Lee JB, Pyo K-H, Kim HR. Role and function of O-GlcNAcylation in cancer. Cancers. 2021;13:5365.
Chatham JC, Zhang J, Wende AR. Role of O-linked N-acetylglucosamine protein modification in cellular (patho)physiology. Physiol Rev. 2021;101:427–93.
Zhou P, Chang WY, Gong DA, Huang LY, Liu R, Liu Y, et al. O-GlcNAcylation of SPOP promotes carcinogenesis in hepatocellular carcinoma. Oncogene. 2023;42:725–36.
Dang F, Nie L, Wei W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021;28:427–38.
Obaya AJ, Sedivy JM. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59:126–42.
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2–Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–3.
Reed SI. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol. 2003;4:855–64.
Levine ZG, Potter SC, Joiner CM, Fei GQ, Nabet B, Sonnett M, et al. Mammalian cell proliferation requires noncatalytic functions of O-GlcNAc transferase. Proc Natl Acad Sci USA. 2021;118:e2016778118.
Zhu Q, Zhou H, Wu L, Lai Z, Geng D, Yang W, et al. O-GlcNAcylation promotes pancreatic tumor growth by regulating malate dehydrogenase 1. Nat Chem Biol. 2022;18:1087–95.
Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80.
Nie H, Ju H, Fan J, Shi X, Cheng Y, Cang X, et al. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat Commun. 2020;11:36.
Yang Y, Yan Y, Yin J, Tang N, Wang K, Huang L, et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner. Signal Transduct Target Ther. 2023;8:63.
Masclef L, Dehennaut V, Mortuaire M, Schulz C, Leturcq M, Lefebvre T, et al. Cyclin D1 stability is partly controlled by O-GlcNAcylation. Front Endocrinol. 2019;10:106.
Wu J, Tan Z, Li H, Lin M, Jiang Y, Liang L, et al. Melatonin reduces proliferation and promotes apoptosis of bladder cancer cells by suppressing O‐GlcNAcylation of cyclin‐dependent‐like kinase 5. J Pineal Res. 2021;71:e12765.
Qiu H, Liu F, Tao T, Zhang D, Liu X, Zhu G, et al. Modification of p27 with O-linked N-acetylglucosamine regulates cell proliferation in hepatocellular carcinoma. Mol Carcinog. 2017;56:258–71.
Fagan-Solis KD, Pentecost BT, Gozgit JM, Bentley BA, Marconi SM, Otis CN, et al. SKP2 overexpression is associated with increased serine 10 phosphorylation of p27 (pSer10p27) in triple-negative breast cancer. J Cell Physiol. 2014;229:1160–9.
Zhao H, Lu Z, Bauzon F, Fu H, Cui J, Locker J, et al. p27T187A knockin identifies Skp2/Cks1 pocket inhibitors for advanced prostate cancer. Oncogene. 2017;36:60–70.
Masuda T, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, et al. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819–25.
Westermann F, Henrich K-O, Wei JS, Lutz W, Fischer M, König R, et al. High Skp2 expression characterizes high-risk neuroblastomas independent of MYCN status. Clin Cancer Res J Am Assoc Cancer Res. 2007;13:4695–703.
Wang H, Luo J, Tian X, Xu L, Zhai Z, Cheng M, et al. DNAJC5 promotes hepatocellular carcinoma cells proliferation though regulating SKP2 mediated p27 degradation. Biochim Biophys Acta Mol Cell Res. 2021;1868:118994.
Wang S-T, Ho HJ, Lin J-T, Shieh J-J, Wu C-Y. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017;8:e2626.
Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell. 2023;11:1177–88.
von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–200.
Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JMA, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci USA. 2005;102:1649–54.
Asmamaw MD, Zhang LR, Liu HM, Shi XJ, Liu Y. Skp2 is a novel regulator of LSD1 expression and function in gastric cancer. Genes Dis. 2023;10:2267–9.
Chan C-H, Li C-F, Yang W-L, Gao Y, Lee S-W, Feng Z, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–111.
Yao F, Zhou Z, Kim J, Hang Q, Xiao Z, Ton BN, et al. SKP2- and OTUD1-regulated non-proteolytic ubiquitination of YAP promotes YAP nuclear localization and activity. Nat Commun. 2018;9:2269.
Wang I-C, Chen Y-J, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead Box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875–94.
Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–42.
Rodier G, Coulombe P, Tanguay P-L, Boutonnet C, Meloche S. Phosphorylation of Skp2 regulated by CDK2 and Cdc14B protects it from degradation by APC(Cdh1) in G1 phase. EMBO J. 2008;27:679–91.
Han F, Li C-F, Cai Z, Zhang X, Jin G, Zhang W-N, et al. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat Commun. 2018;9:4728.
Lin H-K, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan C-H, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–32.
Inuzuka H, Gao D, Finley LWS, Yang W, Wan L, Fukushima H, et al. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–93.
Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83.
Abukhdeir AM, Park BH. p21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19.
Roy S, Gu M, Ramasamy K, Singh RP, Agarwal C, Siriwardana S, et al. P21/Cip1 and P27/Kip1 are essential molecular targets of inositol hexaphosphate for its antitumor efficacy against prostate cancer. Cancer Res. 2009;69:1166–73.
García-Osta A, Dong J, Moreno-Aliaga MJ, Ramirez MJ. p27, The cell cycle and Alzheimer´s disease. Int J Mol Sci. 2022;23:1211.
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52.
Chan C-H, Morrow JK, Li C-F, Gao Y, Jin G, Moten A, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154:556–68.
Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, Mendy D, et al. Targeting the p27 E3 ligase SCFSkp2 results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–9.
Rico-Bautista E, Yang C-C, Lu L, Roth GP, Wolf DA. Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol. 2010;8:153.
Tuo L, Xiang J, Pan X, Hu J, Tang H, Liang L, et al. PCK1 negatively regulates cell cycle progression and hepatoma cell proliferation via the AMPK/p27Kip1 axis. J Exp Clin Cancer Res. 2019;38:50.
Xiang J, Chen C, Liu R, Gou D, Chang L, Deng H, et al. Gluconeogenic enzyme PCK1 deficiency promotes CHK2 O-GlcNAcylation and hepatocellular carcinoma growth upon glucose deprivation. J Clin Invest. 2021;131:e144703.
Acknowledgements
We are grateful that Prof. Tongchuan He (University of Chicago, USA) kindly provided the AdEasy system. We also thank Prof. Ding Xue (Tsinghua University, Beijing, China) for supplying the CRISPR/Cas9 system. This work was supported by the China National Natural Science Foundation (Grant nos. 82272975, 82073251, 82072286, 82304288), the National Key Research and Development Program of China (2023YFC23068), the Innovative and Entrepreneurial Team of Chongqing Talents Plan, Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau, 2023DBXM007), the Future Medical Youth Innovation Team of Chongqing Medical University (W0036, W0101), Senior Medical Talents Program of Chongqing for Young and Middle-aged, and the Kuanren talents and DengFeng program of the second affiliated hospital of Chongqing Medical University.
Author information
Authors and Affiliations
Contributions
LYH, NT, and KW designed the experiments; ZQF JXY and ZRZ performed and analyzed the data; ZQF, JXY, ZRZ, and ZC contributed materials and data, and assisted in data analysis; ZQF, JXY, LYH, NT, and KW wrote and edited the paper. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol for clinical patient samples was approved by the Medical Ethics Committee of Chongqing Medical University (reference number: 2023076). Experimental animals were handled according to guidelines approved by the Institutional Animal Care and Use Committee of Chongqing Medical University (approval number: 20230188). A pathogen-free environment was provided to keep all mice in the Laboratory Animal Center of the Chongqing Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, Z., Yin, J., Zhang, Z. et al. O-GlcNAcylation of E3 ubiquitin ligase SKP2 promotes hepatocellular carcinoma proliferation. Oncogene 43, 1149–1159 (2024). https://doi.org/10.1038/s41388-024-02977-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02977-7