Abstract
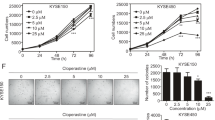
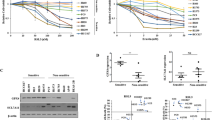
Targeted therapy attempts are needed to enhance esophageal squamous cell carcinoma (ESCC) patients’ overall survival and satisfaction of life. Nuclear factor erythroid 2-related factor 2 (NRF2), as a high-confidence cancer driver gene, controls the antioxidant response, metabolic balance and redox homeostasis in cancer and is regarded as a potent molecular target for cancer treatment. Here, we attempted to find a new NRF2 inhibitor and study the underlying molecular mechanism in ESCC. We found that up-regulated NRF2 protein was negatively correlated with patient prognosis and promoted tumor proliferation in ESCC. Moreover, Pizotifen malate (PZM), a FDA-approved medication, bound to the Neh1 domain of NRF2 and prevented NRF2 protein binding to the ARE motif of target genes, suppressing transcription activity of NRF2. PZM treatment suppressed tumor development in ESCC PDX model by inducing ferroptosis via down-regulating the transcription of GPX4, GCLC, ME1 and G6PD. Our study illustrates that the over expression of NRF2 indicates poor prognosis and promotes tumor proliferation in ESCC. PZM, as a novel NRF2 inhibitor, inhibits the tumor growth by inducing ferroptosis and elucidates a potent NRF2-based therapy strategy for patients with ESCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Yang YM, Hong P, Xu WW, He QY, Li B Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther 2020;5. https://doi.org/10.1038/S41392-020-00323-3.
Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1. Cell. 2019;178:316–29.e18. https://doi.org/10.1016/J.CELL.2019.06.003.
Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, Leboeuf SE, et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 2017;23:1362–8. https://doi.org/10.1038/NM.4407.
Tsai WC, Hueng DY, Lin CR, Yang TCK, Gao HW Nrf2 Expressions Correlate with WHO Grades in Gliomas and Meningiomas. Int J Mol Sci 2016;17. https://doi.org/10.3390/IJMS17050722.
Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21–43. https://doi.org/10.1016/J.CCELL.2018.03.022.
Mukhopadhyay S, Goswami D, Adiseshaiah PP, Burgan W, Yi M, Guerin TM, et al. Undermining Glutaminolysis Bolsters Chemotherapy While NRF2 Promotes Chemoresistance in KRAS-Driven Pancreatic Cancers. Cancer Res. 2020;80:1630–43. https://doi.org/10.1158/0008-5472.CAN-19-1363.
Singh A, Daemen A, Nickles D, Jeon SM, Foreman O, Sudini K, et al. NRF2 Activation Promotes Aggressive Lung Cancer and Associates with Poor Clinical Outcomes. Clin Cancer Res. 2021;27:877–88. https://doi.org/10.1158/1078-0432.CCR-20-1985.
Ma S, Paiboonrungruan C, Yan T, Williams KP, Major MB, Chen XL. Targeted therapy of esophageal squamous cell carcinoma: The NRF2 signaling pathway as target. Ann N Y Acad Sci 2018;1434. https://doi.org/10.1111/nyas.13681.
Harder B, Tian W, La Clair JJ, Tan AC, Ooi A, Chapman E, et al. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog. 2017;56:1493–500. https://doi.org/10.1002/MC.22609.
Mailankody S, Prasad V. Five Years of Cancer Drug Approvals: Innovation, Efficacy, and Costs. JAMA Oncol. 2015;1:539–40. https://doi.org/10.1001/JAMAONCOL.2015.0373.
Xia D, Zhang XR, Ma YL, Zhao ZJ, Zhao R, Wang YY Nrf2 promotes esophageal squamous cell carcinoma (ESCC) resistance to radiotherapy through the CaMKIIα-associated activation of autophagy. Cell Biosci 2020;10. https://doi.org/10.1186/S13578-020-00456-6.
Li B, Yu Y, Jiang Y, Zhao L, Li A, Li M, et al. Cloperastine inhibits esophageal squamous cell carcinoma proliferation in vivo and in vitro by suppressing mitochondrial oxidative phosphorylation. Cell Death Discov 2021;7. https://doi.org/10.1038/S41420-021-00509-W.
Przegaliński E, Baran L, Palider W, Siwanowicz J. The central action of pizotifen. Psychopharmacol (Berl). 1979;62:295–300. https://doi.org/10.1007/BF00431961.
Cloer EW, Goldfarb D, Schrank TP, Weissman BE, Major MB. Nrf2 activation in cancer: From DNA to protein. Cancer Res. 2019;79:889–98. https://doi.org/10.1158/0008-5472.CAN-18-2723.
Xu F, Huang X, Li Y, Chen Y, Lin L. m6A-related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with LUAD. Mol Ther - Nucleic Acids. 2021;24:780–91. https://doi.org/10.1016/J.OMTN.2021.04.003.
Liu F, Wu Q, Han W, Laster K, Hu Y, Ma F, et al. Targeting integrin αvβ3 with indomethacin inhibits patient-derived xenograft tumour growth and recurrence in oesophageal squamous cell carcinoma. Clin Transl Med 2021;11. https://doi.org/10.1002/ctm2.548.
Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–203.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. https://doi.org/10.1016/j.cell.2012.03.042.
Lin HN, Chen LQ, Shang QX, Yuan Y, Yang YS. A meta-analysis on surgery with or without postoperative radiotherapy to treat squamous cell esophageal carcinoma. Int J Surg. 2020;80:184–91. https://doi.org/10.1016/j.ijsu.2020.06.046.
Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr). 2020;43:195–209. https://doi.org/10.1007/S13402-019-00488-2.
Kim J, Bowlby R, Mungall AJ, Robertson AG, Odze RD, Cherniack AD, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–74. https://doi.org/10.1038/NATURE20805.
Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA. 2011;108:1433–8. https://doi.org/10.1073/PNAS.1014275108.
Choi EJ, Jung BJ, Lee SH, Yoo HS, Shin EA, Ko HJ, et al. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene. 2017;36:5285–95. https://doi.org/10.1038/ONC.2017.153.
Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88:101–7. https://doi.org/10.1016/J.FREERADBIOMED.2015.05.034.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. https://doi.org/10.1038/S41586-021-03819-2.
Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucl Acids Res 2021. https://doi.org/10.1093/NAR/GKAB1061.
Hirotsu Y, Katsuoka F, Funayama R, Nagashima T, Nishida Y, Nakayama K, et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucl Acids Res. 2012;40:10228–39. https://doi.org/10.1093/NAR/GKS827.
Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li Z, et al. The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase. Cell. 2019;178:807–09.e21. https://doi.org/10.1016/j.cell.2019.07.031.
Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 2012 128. 2012;12:564–71. https://doi.org/10.1038/nrc3278.
Stockwell BR, Jiang X. A Physiological Function for Ferroptosis in Tumor Suppression by the Immune System. Cell Metab. 2019;30:14–5. https://doi.org/10.1016/J.CMET.2019.06.012.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–85. https://doi.org/10.1016/J.CELL.2017.09.021.
Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30:e1704007 https://doi.org/10.1002/ADMA.201704007.
Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell. 2019;35:830–49. https://doi.org/10.1016/J.CCELL.2019.04.002.
Chen P, Li X, Zhang R, Liu S, Xiang Y, Zhang M, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107–19. https://doi.org/10.7150/THNO.44705.
Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S, et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct Target Ther 2020;5. https://doi.org/10.1038/S41392-020-0149-3.
Hong T, Lei G, Chen X, Li H, Zhang X, Wu N, et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol 2021;42. https://doi.org/10.1016/J.REDOX.2021.101928.
Wu Y, Yu C, Luo M, Cen C, Qiu J, Zhang S, et al. Ferroptosis in Cancer Treatment: Another Way to Rome. Front Oncol 2020;10. https://doi.org/10.3389/FONC.2020.571127.
Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278:12029–38. https://doi.org/10.1074/JBC.M211558200.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. https://doi.org/10.1016/J.CELL.2013.12.010.
Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E, et al. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021;33:174–89.e7. https://doi.org/10.1016/J.CMET.2020.12.007.
Hamai Y, Hihara J, Emi M, Furukawa T, Ibuki Y, Yamakita I, et al. Treatment Outcomes and Prognostic Factors After Recurrence of Esophageal Squamous Cell carcinoma 2017. https://doi.org/10.1007/s00268-017-4430-8.
Luis G, Godfroid A, Nishiumi S, Cimino J, Blacher S, Maquoi E, et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol 2021;43. https://doi.org/10.1016/J.REDOX.2021.102006.
Kerins MJ, Ooi A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid Redox Signal. 2018;29:1756–73. https://doi.org/10.1089/ARS.2017.7176.
Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, Lloyd B, et al. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer 2011;10. https://doi.org/10.1186/1476-4598-10-37.
Zhang HS, Zhang ZG, Du GY, Sun HL, Liu HY, Zhou Z, et al. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med. 2019;23:3451–63. https://doi.org/10.1111/JCMM.14241.
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu C, et al. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl Med 2021;19. https://doi.org/10.1186/S12967-021-03042-7.
Briars GL, Travis SE, Anand B, Kelly AM. Weight gain with pizotifen therapy. Arch Dis Child. 2008;93:590–3. https://doi.org/10.1136/ADC.2007.128611.
Li Z, Li X, He X, Jia X, Zhang X, Lu B, et al. Proteomics Reveal the Inhibitory Mechanism of Levodopa Against Esophageal Squamous Cell Carcinoma. Front Pharm. 2020;11:1–11. https://doi.org/10.3389/fphar.2020.568459.
Xie Y, Zhang J, Lu B, Bao Z, Zhao J, Lu X Mefloquine Inhibits Esophageal Squamous Cell Carcinoma Tumor Growth by Inducing Mitochondrial Autophagy 2020;10:1–13. https://doi.org/10.3389/fonc.2020.01217.
Acknowledgements
We’d like to express our gratitude to Dr. Fred Bogott for the language revision. This work was supported by the National Natural Science Foundations of China (No. 81872335), National Natural Science Youth Foundation (No. 81902486), the Natural Science Foundation of Henan (No. 161100510300), the Central Plains Science and Technology Innovation Leading Talents (No. 224200510015), the Science and Technology Project of Henan Province (No. 212102310187), National Natural Science Foundation of China (32100532), China Postdoctoral Science Foundation (2021M692936), the Supporting Plan of Zhengzhou University Youth Innovation Team (32320442).
Author information
Authors and Affiliations
Contributions
Conceptualization: KL, ZD and YJ. Data curation: XH. Formal analysis: YZ. Funding acquisition: KL, YG, and YJ. Investigation: XH, YZ, YY, ML, WC, XZ, and LD. Methodology: XH and YZ. Project administration: ZD, KL, and YJ. Resources: ZD, KL, and YJ. Software: XH and YG. Supervision: KL and YJ. Validation: XH and YZ. Visualization: XH, JZ, HZ, and HG. Roles/Writing-original draft: XH and YJ. Writing-review and editing: KL and YJ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, X., Zhou, Y., Chen, W. et al. Repurposed pizotifen malate targeting NRF2 exhibits anti-tumor activity through inducing ferroptosis in esophageal squamous cell carcinoma. Oncogene 42, 1209–1223 (2023). https://doi.org/10.1038/s41388-023-02636-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02636-3
This article is cited by
-
Domperidone inhibits cell proliferation via targeting MEK and CDK4 in esophageal squamous cell carcinoma
Cancer Cell International (2024)