Abstract
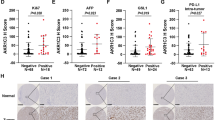
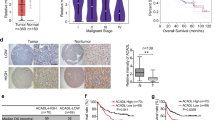
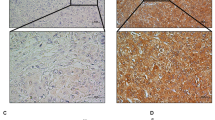
Ether-à-go-go-1 (EAG1), one of the potassium channels, is involved in various physiological processes and plays an important role in the tumorigenesis of many kinds of cancer. EAG1 is highly expressed in hepatocarcinoma cells and is closely related to clinical prognosis, but the molecular mechanism remains elusive. In this study, we verified that EAG1 promotes the proliferation of hepatocellular carcinoma (HCC) both in vitro and in vivo. It promotes cell cycle progression by inhibiting the ubiquitination of SKP2. In addition, EAG1 promotes the migration and invasion of HCC by promoting cell pseudopod formation. Furthermore, in a high-pressure plasmid-injected mouse liver orthotopic carcinoma model, astemizole, an EAG family blocker, can significantly inhibit the formation of liver cancer. Meanwhile, liver-specific EAG1 knockout mice show resistance to hepatocarcinogenesis. This research demonstrated that EAG1 plays an important role in the progression of HCC, and could be a potential therapeutic target for HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–9.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–58.
El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73.e1261.
Stotz M, Gerger A, Haybaeck J, Kiesslich T, Bullock MD, Pichler M. Molecular Targeted Therapies in Hepatocellular Carcinoma: Past, Present and Future. Anticancer Res. 2015;35:5737–44.
Wickenden A. K(+) channels as therapeutic drug targets. Pharm Ther. 2002;94:157–82.
Haitin Y, Carlson AE, Zagotta WN. The structural mechanism of KCNH-channel regulation by the eag domain. Nature. 2013;501:444–8.
Diaz L, Ceja-Ochoa I, Restrepo-Angulo I, Larrea F, Avila-Chavez E, Garcia-Becerra R, et al. Estrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expression. Cancer Res. 2009;69:3300–7.
Liu GX, Yu YC, He XP, Ren SN, Fang XD, Liu F, et al. Expression of eag1 channel associated with the aggressive clinicopathological features and subtype of breast cancer. Int J Clin Exp Pathol. 2015;8:15093–9.
de Guadalupe Chavez-Lopez M, Perez-Carreon JI, Zuniga-Garcia V, Diaz-Chavez J, Herrera LA, Caro-Sanchez CH, et al. Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumour Biol. 2015;36:6149–58.
Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39–48.
Wang X, Chen Y, Zhang Y, Guo S, Mo L, An H, et al. Eag1 Voltage-Dependent Potassium Channels: structure, Electrophysiological Characteristics, and Function in Cancer. J Membr Biol. 2017;250:123–32.
de Guadalupe Chavez-Lopez M, Hernandez-Gallegos E, Vazquez-Sanchez AY, Gariglio P, Camacho J. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int J Gynecol Cancer. 2014;24:824–8.
Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol. 2014;184:912–23.
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–83.
Liu YT, Tseng TC, Soong RS, Peng CY, Cheng YH, Huang SF, et al. A novel spontaneous hepatocellular carcinoma mouse model for studying T-cell exhaustion in the tumor microenvironment. J Immunother Cancer. 2018;6:144.
Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, et al. Single tumor-initiating cells evade immune clearance by recruiting type II macrophages. Genes Dev. 2017;31:247–59.
Garcia-Quiroz J, Camacho J. Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med Chem. 2011;11:307–14.
Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323–33.
Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–9.
Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM. Involvement of F-BOX proteins in progression and development of human malignancies. Semin Cancer Biol. 2016;36:18–32.
Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci USA. 1998;95:11324–9.
Wei Z, Jiang X, Qiao H, Zhai B, Zhang L, Zhang Q, et al. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cell Signal. 2013;25:931–8.
Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98:5043–8.
Signoretti S, Di Marcotullio L, Richardson A, Ramaswamy S, Isaac B, Rue M, et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Investig. 2016;126:4387.
Chen TP, Chen CM, Chang HW, Wang JS, Chang WC, Hsu SI, et al. Increased expression of SKP2 and phospho-MAPK/ERK1/2 and decreased expression of p27 during tumor progression of cervical neoplasms. Gynecol Oncol. 2007;104:516–23.
Yoshida Y, Ninomiya K, Hamada H, Noda M. Involvement of the SKP2-p27(KIP1) pathway in suppression of cancer cell proliferation by RECK. Oncogene. 2012;31:4128–38.
Vaiana SM, Manno M, Emanuele A, Palma-Vittorelli MB, Palma MU. The role of solvent in protein folding and in aggregation. J Biol Phys. 2001;27:133–45.
Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428.
Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5.
Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278:35857–60.
Sheterline P, Clayton J, Sparrow J. Actin. Protein Profile. 1995;2:1–103.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81272675 and 81870434) to PS, the Key Research and Development Plan of Zhejiang Province (2020C04003) to PS, the National S&T Major Project (2017ZX10203205) to SZ.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, J., Xuan, Z., Song, W. et al. EAG1 enhances hepatocellular carcinoma proliferation by modulating SKP2 and metastasis through pseudopod formation. Oncogene 40, 163–176 (2021). https://doi.org/10.1038/s41388-020-01522-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01522-6
This article is cited by
-
PTEN-related risk classification models for predicting prognosis and immunotherapy response of hepatocellular carcinoma
Discover Oncology (2023)
-
Intracellular hemin is a potent inhibitor of the voltage-gated potassium channel Kv10.1
Scientific Reports (2022)
-
Targeting anillin inhibits tumorigenesis and tumor growth in hepatocellular carcinoma via impairing cytokinesis fidelity
Oncogene (2022)