Abstract
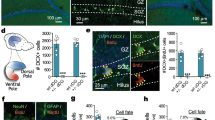
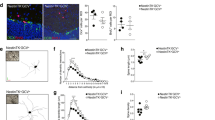
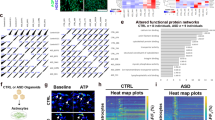
Adult cytogenesis, the continuous generation of newly-born neurons (neurogenesis) and glial cells (gliogenesis) throughout life, is highly impaired in several neuropsychiatric disorders, such as Major Depressive Disorder (MDD), impacting negatively on cognitive and emotional domains. Despite playing a critical role in brain homeostasis, the importance of gliogenesis has been overlooked, both in healthy and diseased states. To examine the role of newly formed glia, we transplanted Glial Restricted Precursors (GRPs) into the adult hippocampal dentate gyrus (DG), or injected their secreted factors (secretome), into a previously validated transgenic GFAP-tk rat line, in which cytogenesis is transiently compromised. We explored the long-term effects of both treatments on physiological and behavioral outcomes. Grafted GRPs reversed anxiety-like deficits and demonstrated an antidepressant-like effect, while the secretome promoted recovery of only anxiety-like behavior. Furthermore, GRPs elicited a recovery of neurogenic and gliogenic levels in the ventral DG, highlighting the unique involvement of these cells in the regulation of brain cytogenesis. Both GRPs and their secretome induced significant alterations in the DG proteome, directly influencing proteins and pathways related to cytogenesis, regulation of neural plasticity and neuronal development. With this work, we demonstrate a valuable and specific contribution of glial progenitors to normalizing gliogenic levels, rescuing neurogenesis and, importantly, promoting recovery of emotional deficits characteristic of disorders such as MDD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Data files).
References
Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7.
Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–81.
Lucassen PJ, Fitzsimons CP, Salta E, Maletic-Savatic M. Adult neurogenesis, human after all (again): Classic, optimized, and future approaches. Behav Brain Res. 2020;381:112458.
Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23:25–30.
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, et al. Human Hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599.e5.
Toda T, Parylak SL, Linker SB, Gage FH. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry. 2019;24:67–87.
Murano T, Nakajima R, Nakao A, Hirata N, Amemori S, Murakami A, et al. Multiple types of navigational information are independently encoded in the population activities of the dentate gyrus neurons. Proc Natl Acad Sci. 2022;119:e2106830119.
Abrous DN, Koehl M, Lemoine M. A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology. Mol Psychiatry. 2022;27:383–402.
Surget A, Belzung C. Adult hippocampal neurogenesis shapes adaptation and improves stress response: a mechanistic and integrative perspective. Mol Psychiatry. 2022;27:403–21.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
Tartt AN, Mariani MB, Hen R, Mann JJ, Boldrini M. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27:2689–99.
Alves ND, Correia JS, Patrício P, Mateus-Pinheiro A, Machado-Santos AR, Loureiro-Campos E, et al. Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression. Transl Psychiatry. 2017;7:e1058.
Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25:530–43.
Martins-Macedo J, Salgado AJ, Gomes ED, Pinto L. Adult brain cytogenesis in the context of mood disorders: From neurogenesis to the emergent role of gliogenesis. Neurosci Biobehav Rev. 2021;131:411–28.
Mateus-Pinheiro A, Pinto L, Bessa JM, Morais M, Alves ND, Monteiro S, et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry. 2013;3:e210.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat Hippocampus. J Neurosci. 2000;20:9104–10.
Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in Hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–96.
Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 2011;16:738–50.
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–88.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109.
Gałecki P, Samochowiec J, Mikułowska M, Szulc A. Treatment-resistant depression in Poland—epidemiology and treatment. J Clin Med. 2022;11:480.
Fugger G, Bartova L, Fabbri C, Fanelli G, Dold M, Swoboda MMM, et al. The sociodemographic and clinical profile of patients with major depressive disorder receiving SSRIs as first-line antidepressant treatment in European countries. Eur Arch Psychiatry Clin Neurosci. 2022;272:715–27.
Bessa JM, Carvalho S, Cunha IB, Fernandes M, Matos-Pires A, Neves R, et al. Treatment-resistant depression in portugal: perspective from psychiatry experts. Front Psychiatry. 2022;13:824919.
Moret C, Isaac M, Briley M. Review: Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J Psychopharmacol. 2009;23:967–74.
Learned S, Graff O, Roychowdhury S, Moate R, Krishnan KR, Archer G, et al. Efficacy, safety, and tolerability of a triple reuptake inhibitor GSK372475 in the treatment of patients with major depressive disorder: two randomized, placebo- and active-controlled clinical trials. J Psychopharmacol. 2012;26:653–62.
Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, Tollefson GD, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin. 2007;23:401–16.
Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression∗∗See accompanying Editorial, in this issue. Biol Psychiatry. 1999;45:1085–98.
Czéh B, Fuchs E, Flügge G. Altered glial plasticity in animal models for mood disorders. Curr Drug Targets. 2013;14:1249–61.
Czéh B, di Benedetto B. Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol. 2013;23:171–85.
Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–80.
Czéh B, Müller-Keuker JIH, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503.
Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44.
Horner PJ, Palmer TD. New roles for astrocytes: The nightlife of an ‘astrocyte’. La vida loca! Trends Neurosci. 2003;26:597–603.
Machado-Santos AR, Alves ND, Araújo B, Correia JS, Patrício P, Mateus-Pinheiro A, et al. Astrocytic plasticity at the dorsal dentate gyrus on an animal model of recurrent depression. Neuroscience. 2021;454:94–104.
Machado-Santos AR, Loureiro-Campos E, Patrício P, Araújo B, Alves ND, Mateus-Pinheiro A, et al. Beyond new neurons in the adult hippocampus: imipramine acts as a pro-astrogliogenic factor and rescues cognitive impairments induced by stress exposure. Cells. 2022;11:390.
Mateus-Pinheiro A, Patrício P, Alves ND, Martins-Macedo J, Caetano I, Silveira-Rosa T, et al. Hippocampal cytogenesis abrogation impairs inter-regional communication between the hippocampus and prefrontal cortex and promotes the time-dependent manifestation of emotional and cognitive deficits. Mol Psychiatry. 2021;26:7154–66.
Groves JO, Leslie I, Huang GJ, McHugh SB, Taylor A, Mott R, et al. Ablating adult neurogenesis in the rat has no effect on spatial processing: evidence from a novel pharmacogenetic model. PLoS Genet. 2013;9:e1003718.
Martins‐Macedo J, Lepore AC, Domingues HS, Salgado AJ, Gomes ED, Pinto L. Glial restricted precursor cells in central nervous system disorders: Current applications and future perspectives. Glia. 2021;69:513–31.
Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63.
Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci. 1999;19:1049–61.
Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–42.
Jordan WH. Book review: the rat brain in stereotaxic coordinates. Vet Pathol. 2006;43:86–7.
Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2.
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57.
Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:e21800.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
Chen T, Liu Y, Huang L. ImageGP: An easy‐to‐use data visualization web server for scientific researchers. iMeta. 2022;1:e5.
Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–60.
Hattiangady B, Shuai B, Cai J, Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging Hippocampus. Stem Cells. 2007;25:2104–17.
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–61.
Koyanagi I, Akers K, Vergara P, Srinivasan S, Sakurai T, Sakaguchi M. Memory consolidation during sleep and adult hippocampal neurogenesis. Neural Regen Res. 2019;14:20.
O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–5.
Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19:9054–62.
Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci. 1995;92:9697–701.
Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–67.
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci. 2002;99:10825–30.
Weeden CSS, Roberts JM, Kamm AM, Kesner RP. The role of the ventral dentate gyrus in anxiety-based behaviors. Neurobiol Learn Mem. 2015;118:143–9.
Houser CR, Peng Z, Wei X, Huang CS, Mody I. Mossy cells in the dorsal and ventral dentate gyrus differ in their patterns of axonal projections. J Neurosci. 2021;41:991–1004.
Fredes F, Silva MA, Koppensteiner P, Kobayashi K, Joesch M, Shigemoto R. Ventro-dorsal hippocampal pathway gates novelty-induced contextual memory formation. Curr Biol. 2021;31:25–38.e5.
Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–91.
Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623.
Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715.
Sun D, Milibari L, Pan JX, Ren X, Yao LL, Zhao Y, et al. Critical roles of embryonic born dorsal dentate granule neurons for activity-dependent increases in BDNF, adult hippocampal neurogenesis, and antianxiety-like behaviors. Biol Psychiatry. 2021;89:600–14.
Botterill JJ, Gerencer KJ, Vinod KY, Alcantara‐Gonzalez D, Scharfman HE. Dorsal and ventral mossy cells differ in their axonal projections throughout the dentate gyrus of the mouse hippocampus. Hippocampus. 2021;31:522–39.
Jeong M, Jang J-H, Oh S-J, Choe Y-S, Park J, Lee J, et al. Intrinsic heterogeneity of mossy cells mediates the differential crosstalk between the dorsal and ventral hippocampus. Preprint (Version 1) available at Research Square https://doi.org/10.21203/rs.3.rs-92512/v1. 2020.
Botterill JJ, Vinod KY, Gerencer KJ, Teixeira CM, LaFrancois JJ, Scharfman HE. Bidirectional regulation of cognitive and anxiety-like behaviors by dentate gyrus mossy cells in male and female mice. J Neurosci. 2021;41:2475–95.
Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science (1979). 2005;307:1324–8.
Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–26.
Blundell J, Tabuchi K, Bolliger MF, Blaiss CA, Brose N, Liu X, et al. Increased anxiety-like behavior in mice lacking the inhibitory synapse cell adhesion molecule neuroligin 2. Genes Brain Behav. 2009;8:114–26.
Babaev O, Botta P, Meyer E, Müller C, Ehrenreich H, Brose N, et al. Neuroligin 2 deletion alters inhibitory synapse function and anxiety-associated neuronal activation in the amygdala. Neuropharmacology. 2016;100:56–65.
Williamson MA. Aryl hydrocarbon receptor expression and activity in cerebellar granule neuroblasts: implications for development and dioxin neurotoxicity. Toxicol Sci. 2004;83:340–8.
Latchney SE, Hein AM, O’Banion MK, DiCicco-Bloom E, Opanashuk LA. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem. 2013;125:430–45.
de la Parra J, Cuartero MI, Pérez-Ruiz A, García-Culebras A, Martín R, Sánchez-Prieto J, et al. AhR deletion promotes aberrant morphogenesis and synaptic activity of adult-generated granule neurons and impairs hippocampus-dependent memory. eNeuro. 2018;5:ENEURO.0370-17.2018.
Kleindienst A, McGinn MJ, Harvey HB, Colello RJ, Hamm RJ, Bullock MR. Enhanced hippocampal neurogenesis by intraventricular s100b infusion is associated with improved cognitive recovery after traumatic brain injury. J Neurotrauma. 2005;22:645–55.
Hashikawa N, Utaka Y, Ogawa T, Tanoue R, Morita Y, Yamamoto S, et al. HSP105 prevents depression-like behavior by increasing hippocampal brain-derived neurotrophic factor levels in mice. Sci Adv. 2017;3:e1603014.
Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589–97.
Sharifi K, Morihiro Y, Maekawa M, Yasumoto Y, Hoshi H, Adachi Y, et al. FABP7 expression in normal and stab-injured brain cortex and its role in astrocyte proliferation. Histochem Cell Biol. 2011;136:501–13.
Frischknecht R, Seidenbecher CI. Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol. 2012;44:1051–4.
Mani S, Shen Y, Schaefer J, Meiri KF. Failure to express GAP-43 during neurogenesis affects cell cycle regulation and differentiation of neural precursors and stimulates apoptosis of neurons. Mol Cell Neurosci. 2001;17:54–66.
Geissler M, Gottschling C, Aguado A, Rauch U, Wetzel CH, Hatt H, et al. Primary hippocampal neurons, which lack four crucial extracellular matrix molecules, display abnormalities of synaptic structure and function and severe deficits in perineuronal net formation. J Neurosci. 2013;33:7742–55.
Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364–74.
Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem [Internet]. 2007;0:070214184024009-??? Available from: https://doi.org/10.1111/j.1471-4159.2006.04319.x.
Lopes S, Vaz-Silva J, Pinto V, Dalla C, Kokras N, Bedenk B, et al. Tau protein is essential for stress-induced brain pathology. Proc Natl Acad Sci. 2016;113:E3755-E3763.
Wen G, Yao H, Li Y, Ding R, Ren X, Tan Y, et al. Regulation of tau protein on the antidepressant effects of ketamine in the chronic unpredictable mild stress model. Front Psychiatry. 2019;10:287.
Joy T, Rao M, Madhyastha S. N-acetyl cysteine supplement minimize tau expression and neuronal loss in animal model of Alzheimer’s disease. Brain Sci. 2018;8:185.
Janke KL, Cominski TP, Kuzhikandathil EV, Servatius RJ, Pang KCH. Investigating the role of hippocampal bdnf in anxiety vulnerability using classical eyeblink conditioning. Front Psychiatry. 2015;6:106.
JiaWen W, Hong S, ShengXiang X, Jing L. Depression- and anxiety-like behaviour is related to BDNF/TrkB signalling in a mouse model of psoriasis. Clin Exp Dermatol. 2018;43:254–61.
Prowse N, Dwyer Z, Thompson A, Fortin T, Elson K, Robeson H, et al. Early life selective knockdown of the TrkB receptor and maternal separation modulates adult stress phenotype. Behav Brain Res. 2020;378:112260.
Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Akhtari AS, Karimi M. Comparison of the adulthood chronic stress effect on hippocampal BDNF signaling in male and female rats. Mol Neurobiol. 2016;53:4026–33.
Marosi K, Kim SW, Moehl K, Scheibye‐Knudsen M, Cheng A, Cutler R, et al. 3‐Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139:769–81.
Acknowledgements
We would like to thank Prof. Itzhak Fisher for providing the transgenic rats to isolate GRPs and Prof. Jonathan Flint for providing the GFAP-tk rat line. L.P. was funded by the Portuguese Foundation for Science and Technology (FCT, 2020.02855.CEECIND, CEECINST/00077/2018/CP1640/CT0003 and https://doi.org/10.54499/2022.02201.PTDC). This work was funded by Nature Research Award for Driving Global Impact – 2019 Brain Sciences (to L.P.). A.J.S. received funding from Prémios Santa Casa Neurociências – Prize Melo e Castro for Spinal Cord Injury Research (MC-04/17; MC-18-2021), and from FCT (POCI-01-0145-FEDER-029206). A.J.R. was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 101003187), by “La Caixa” Foundation (ID 100010434), under the agreement LCF/PR/HR20/52400020, as well as by FCT, under the scope of the project https://doi.org/10.54499/PTDC/MED-NEU/4804/2020 (ENDOPIO). This work has been also funded by National funds, through the FCT – project UIDB/50026/2020 and UIDP/50026/2020. A.C.L. was supported by the National Institute of Neurological Disorders and Stroke (R01NS079702, R01NS110385) and the Yant Family Spinal Cord Regeneration Fund. S.I.A. was funded by FCT (2021.04378.CEECIND and EXPL/BTM-TEC/1407/2021). B.M. was funded by The National Mass Spectrometry Network (POCI-01-0145-FEDER-402-022125 Ref.ROTEIRO/0028/2013) and FCT (UIDB/04539/2020 and UIDP/04539/2020).
Author information
Authors and Affiliations
Contributions
JMM, BA, TSR and PP maintained the GFAP-tk colony, induced the model, performed genotyping and collected wellbeing measures. ACL isolated and provided the GRPs. JMM and EDG conducted all behavioral tests, in vitro experiments and immunohistochemical experiments, performed the analyses and interpreted the results. BA assisted in the immunohistochemical experiments and analyses. SIA performed the proteome experiments. SIA, FGT, JMS, NDA and BM analyzed and interpreted the proteome data. JMM, EDG, AJS and LP designed the study, planned the experiments and wrote the manuscript. JMM, EDG, AJS, AJR, BA, ACL and LP edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martins-Macedo, J., Araújo, B., Anjo, S.I. et al. Glial-restricted precursors stimulate endogenous cytogenesis and effectively recover emotional deficits in a model of cytogenesis ablation. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02490-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02490-z