Abstract
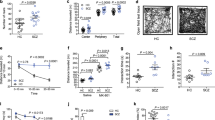
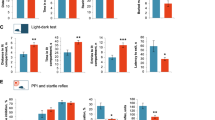
Emerging evidence suggests that the gut microbiota is closely related to psychiatric disorders. However, little is known about the role of the gut microbiota in the development of obsessive-compulsive disorder (OCD). Here, to investigate the contribution of gut microbiota to the pathogenesis of OCD, we transplanted fecal microbiota from first-episode, drug-naive OCD patients or demographically matched healthy individuals into antibiotic-treated specific pathogen-free (SPF) mice and showed that colonization with OCD microbiota is sufficient to induce core behavioral deficits, including abnormal anxiety-like and compulsive-like behaviors. The fecal microbiota was analyzed using 16 S rRNA full-length sequencing, and the results demonstrated a clear separation of the fecal microbiota of mice colonized with OCD and control microbiota. Notably, microbiota from OCD-colonized mice resulted in injured neuronal morphology and function in the mPFC, with inflammation in the mPFC and colon. Unbiased metabolomic analyses of the serum and mPFC region revealed the accumulation of succinic acid (SA) in OCD-colonized mice. SA impeded neuronal activity and induced an inflammatory response in both the colon and mPFC, impacting intestinal permeability and brain function, which act as vital signal mediators in gut microbiota–brain–immune crosstalk. Manipulations of dimethyl malonate (DM) have been reported to exert neuroprotective effects by suppressing the oxidation of accumulated succinic acid, attenuating the downstream inflammatory response and neuronal damage, and can help to partly improve abnormal behavior and reduce neuroinflammation and intestinal inflammation in OCD-colonized mice. We propose that the gut microbiota likely regulates brain function and behaviors in mice via succinic acid signaling, which contributes to the pathophysiology of OCD through gut-brain crosstalk and may provide new insights into the treatment of this disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive [80] in National Genomics Data Center [81], China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA014210, CRA014216) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
References
Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Yang W, Tang Z, Wang X, Ma X, Cheng Y, Wang B, et al. The cost of obsessive-compulsive disorder (OCD) in China: a multi-center cross-sectional survey based on hospitals. Gen Psychiatr. 2021;34:e100632.
Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102:27–47.
Kaplan A, Hollander E. A review of pharmacologic treatments for obsessive-compulsive disorder. Psychiatr Serv. 2003;54:1111–8.
Grassi G, Pallanti S. Current and up-and-coming pharmacotherapy for obsessive-compulsive disorder in adults. Expert Opin Pharmacother. 2018;19:1541–50.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013.
Turna J, Grosman Kaplan K, Anglin R, Patterson B, Soreni N, Bercik P, et al. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: a pilot study. Acta Psychiatr Scand. 2020;142:337–47.
Domènech L, Willis J, Alemany-Navarro M, Morell M, Real E, Escaramís G, et al. Changes in the stool and oropharyngeal microbiome in obsessive-compulsive disorder. Sci Rep. 2022;12:1448.
Scheepers IM, Cryan JF, Bastiaanssen TFS, Rea K, Clarke G, Jaspan HB, et al. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur J Neurosci. 2019;51:1419–27.
Kantak PA, Bobrow DN, Nyby JG. Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav Pharm. 2014;25:71–79.
Messaoudi M, Violle N, Bisson J-F, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256–61.
Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19:77–94.
Zhu Y, Fan Q, Han X, Zhang H, Chen J, Wang Z, et al. Decreased thalamic glutamate level in unmedicated adult obsessive–compulsive disorder patients detected by proton magnetic resonance spectroscopy. J Affect Disord. 2015;178:193–200.
Murgia F, Gagliano A, Tanca MG, Or-Geva N, Hendren A, Carucci S, et al. Metabolomic Characterization of Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). Front Neurosci. 2021;15:645267.
Aoki Y, Aoki A, Suwa H. Reduction of N-acetylaspartate in the medial prefrontal cortex correlated with symptom severity in obsessive-compulsive disorder: meta-analyses of 1H-MRS studies. Transl Psychiatry. 2012;2:e153–e153.e153.
Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity. 2018;49:33–41.e7.
Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol psychiatry. 2019;25:2905–18.
Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–43.
Deacon RMJ. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–4.
Xu P, Grueter BA, Britt JK, McDaniel L, Huntington PJ, Hodge R, et al. Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc Natl Acad Sci USA. 2013;110:10759–64.
Liang X, FitzGerald GA. Timing the microbes: the circadian rhythm of the gut microbiome. J Biol Rhythms. 2017;32:505–15.
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry. 2017;82:472–87.
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5.
Siopi E, Galerne M, Rivagorda M, Saha S, Moigneu C, Moriceau S, et al. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol Psychiatry. 2023;28:3002–12.
Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020;10:186.
Fan S, Guo W, Xiao D, Guan M, Liao T, Peng S, et al. Microbiota-gut-brain axis drives overeating disorders. Cell Metab. 2023;35:2011–.e7.
Wu Y, Zhang Y, Xie B, Abdelgawad A, Chen X, Han M, et al. RhANP attenuates endotoxin-derived cognitive dysfunction through subdiaphragmatic vagus nerve-mediated gut microbiota-brain axis. J Neuroinflammation. 2021;18:300.
Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, et al. Cytokines are markers of the clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol. 2017;24:e00037–17.
Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69.
Kapiki A, Costalos C, Oikonomidou C, Triantafyllidou A, Loukatou E, Pertrohilou V. The effect of a fructo-oligosaccharide supplemented formula on gut flora of preterm infants. Early Hum Dev. 2007;83:335–9.
van Vlies N, Hogenkamp A, Thijssen S, Dingjan GM, Knipping K, Garssen J, et al. Effects of short-chain galacto- and long-chain fructo-oligosaccharides on systemic and local immune status during pregnancy. J Reprod Immunol. 2012;94:161–8.
Ahmari SE, Rauch SL. The prefrontal cortex and OCD. Neuropsychopharmacology. 2022;47:211–24.
Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–55.
Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci. 2017;40:21–49.
Xu J, Pan H, Xie X, Zhang J, Wang Y, Yang G. Inhibiting succinate dehydrogenase by dimethyl malonate alleviates brain damage in a rat model of cardiac arrest. Neuroscience. 2018;393:24–32.
Li Y, Liu Y, Wang C, Xia W-R, Zheng J-Y, Yang J, et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic Biol Med. 2018;126:1–14.
Zhang Y, Cui Y, Cheng Y, Zhu W, Zhang M, Li S, et al. Succinate accumulation contributes to oxidative stress and iron accumulation in pentylenetetrazol-induced epileptogenesis and kainic acid-induced seizure. Neurochem Int. 2021;149:105123.
Fernández-Veledo S, Vendrell J. Gut microbiota-derived succinate: friend or foe in human metabolic diseases? Rev Endocr Metab Disord. 2019;20:439–47.
Jung TD, Jung PS, Raveendran L, Farbod Y, Dvorkin-Gheva A, Sakic B, et al. Changes in gut microbiota during development of compulsive checking and locomotor sensitization induced by chronic treatment with the dopamine agonist quinpirole. Behav Pharm. 2018;29:211–24.
Quagliariello A, Del Chierico F, Russo A, Reddel S, Conte G, Lopetuso LR, et al. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front Microbiol. 2018;9:675.
van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJLM, van Hartskamp J, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9.
Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. 2017;81:708–17.
Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605.
Dries DR, Zhu Y, Brooks MM, Forero DA, Adachi M, Cenik B, et al. Loss of nicastrin from oligodendrocytes results in hypomyelination and schizophrenia with compulsive behavior. J Biol Chem. 2016;291:11647–56.
de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9.
Huang B-L, Wang J-R, Yang X-H, Ren Y-M, Guo H-R. A study on diffusion tensor imaging in patients with untreated first-episode obsessive-compulsive disorder. Quant Imaging Med Surg. 2022;12:1467–74.
Noh HJ, Tang R, Flannick J, O’Dushlaine C, Swofford R, Howrigan D, et al. Integrating evolutionary and regulatory information with a multispecies approach implicates genes and pathways in obsessive-compulsive disorder. Nat Commun. 2017;8:774.
Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900.
Nagarajan N, Jones BW, West PJ, Marc R. Capecchi MRJMp. Corticostriatal circuit defects in Hoxb8 mutant mice. Mol Psychiatry. 2018;23:1868–77.
Sherwin E, Bordenstein SR, Quinn JL, Dinan TG, Cryan JF. Microbiota and the social brain. Science. 2019;366:eaar2016.
Mirzaei R, Bouzari B, Hosseini-Fard SR, Mazaheri M, Ahmadyousefi Y, Abdi M, et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed Pharmacother. 2021;139:111661.
Liu Y-Y, Zhou X-Y, Yang L-N, Wang H-Y, Zhang Y-Q, Pu J-C, et al. Social defeat stress causes depression-like behavior with metabolite changes in the prefrontal cortex of rats. PLoS ONE. 2017;12:e0176725.
Tian L, Pu J, Liu Y, Gui S, Zhong X, Song X, et al. Metabolomic analysis of animal models of depression. Metab Brain Dis. 2020;35:979–90.
Pan J-X, Xia J-J, Deng F-L, Liang W-W, Wu J, Yin B-M, et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry. 2018;8:130.
Qu Y, Zhang K, Pu Y, Chang L, Wang S, Tan Y, et al. Betaine supplementation is associated with the resilience in mice after chronic social defeat stress: a role of brain-gut-microbiota axis. J Affect Disord. 2020;272:66–76.
Hernandez-Baixauli J, Puigbò P, Abasolo N, Palacios-Jordan H, Foguet-Romero E, Suñol D, et al. Alterations in metabolome and microbiome associated with an early stress stage in male wistar rats: a multi-omics approach. Int J Mol Sci. 2021;22:12931.
Liu M-L, Zhang X-T, Du X-Y, Fang Z, Liu Z, Xu Y, et al. Severe disturbance of glucose metabolism in peripheral blood mononuclear cells of schizophrenia patients: a targeted metabolomic study. J Transl Med. 2015;13:226.
Zasłona Z, O’Neill LAJ. Cytokine-like roles for metabolites in immunity. Mol Cell. 2020;78:814–23.
Li H, Tan H, Liu Z, Pan S, Tan S, Zhu Y, et al. Succinic acid exacerbates experimental autoimmune uveitis by stimulating neutrophil extracellular traps formation via SUCNR1 receptor. Br J Ophthalmol. 2023;107:1744–49.
Jiang S-S, Xie Y-L, Xiao X-Y, Kang Z-R, Lin X-L, Zhang L, et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. 2023;31:781–.e9.
Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2018;11:25.
Malan-Müller S, Valles-Colomer M, Palomo T, Leza JC. The gut-microbiota-brain axis in a Spanish population in the aftermath of the COVID-19 pandemic: microbiota composition linked to anxiety, trauma, and depression profiles. Gut Microbes. 2023;15:2162306.
Simpson CA, Mu A, Haslam N, Schwartz OS, Simmons JG. Feeling down? A systematic review of the gut microbiota in anxiety/depression and irritable bowel syndrome. J Affect Disord. 2020;266:429–46.
Shin N-R, Whon TW, Bae J-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503.
Troyer EA, Kohn JN, Ecklu-Mensah G, Aleti G, Rosenberg DR, Hong S. Searching for host immune-microbiome mechanisms in obsessive-compulsive disorder: A narrative literature review and future directions. Neurosci Biobehav Rev. 2021;125:517–34.
Sinhorin VDG, Roehrs C, Pasin JSM, Bellé NAV, Rubin MA, Mello CF. Succinate causes oxidative damage through N-methyl-D-aspartate-mediated mechanisms. Brain Res. 2005;1051:66–71.
Peruzzotti-Jametti L, Bernstock JD, Vicario N, Costa ASH, Kwok CK, Leonardi T, et al. Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell. 2018;22:355–.e13.
Thadepalli H, Gangopadhyay PK, Ansari A, Overturf GD, Dhawan VK, Mandal AK. Rapid differentiation of bacterial meningitides by direct gas-liquid chromatography. J Clin Invest. 1982;69:979–84.
Brockmann K, Bjornstad A, Dechent P, Korenke CG, Smeitink J, Trijbels JMF, et al. Succinate in dystrophic white matter: a proton magnetic resonance spectroscopy finding characteristic for complex II deficiency. Ann Neurol. 2002;52:38–46.
Roehrs C, Garrido-Sanabria ER, Da Silva AC, Faria LC, Sinhorin VDG, Marques RH, et al. Succinate increases neuronal post-synaptic excitatory potentials in vitro and induces convulsive behavior through N-methyl-d-aspartate-mediated mechanisms. Neuroscience. 2004;125:965–71.
Sakamoto M, Benno Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. Int J Syst Evol Microbiol. 2006;56:1599–605.
Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–80.
Sun L, Zhang H, Cao Y, Wang C, Zhao C, Wang H, et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int J Med Sci. 2019;16:1260–70.
Lyte M, Daniels KM, Schmitz-Esser S. Fluoxetine-induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ. 2019;7:e6199.
Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab. 2016;27:752–5.
Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–12.
Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–17.
Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69.
Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574:543–8.
Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The genome sequence archive family: toward explosive data growth and diverse data types. Genom Proteom Bioinform. 2021;19:578–83.
CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022;50:D27–D38.
Acknowledgements
This work was supported by grants from the Shanghai Science and Technology Committee (20XD1423100, 23QA1408300), the National Natural Science Foundation of China (82230045, 82071518, 32271066), the Shanghai Municipal Education Commission (2021-01-07-00-02-E0086), and the Xuhui District Artificial Intelligence Medical Hospital Cooperation Project (2021-005).
Author information
Authors and Affiliations
Contributions
ZW, YDZ, and DDS designed the experiments. YDZ and DDS conducted behavioral, molecular and histological assays with the help of BBL, YL, and SZ. DDS performed electrophysiological recording. JG and LJL assisted in patient recruitment and fecal sample collection. YDZ and DDS performed subsequent bioinformatics analysis of transcriptomic and metabolomics data. YDZ wrote the paper and drafted the manuscript, and DDS revised it. ZW supervised the experiments and reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, YD., Shi, DD., Liao, BB. et al. Human microbiota from drug-naive patients with obsessive-compulsive disorder drives behavioral symptoms and neuroinflammation via succinic acid in mice. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02424-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02424-9