Abstract
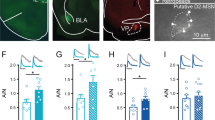
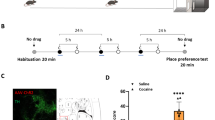
The brain circuit projecting from the ventral tegmental area (VTA) to the nucleus accumbens lateral shell (NAcLat) has a key role in methamphetamine (MA) addiction. As different dopamine (DA) neuron subpopulations in the VTA participate in different neuronal circuits, it is a challenge to isolate these DA neuron subtypes. Using retrograde tracing and Patch-seq, we isolated DA neurons in the VTA-NAcLat circuit in MA-treated mice and performed gene expression profiling. Among the differentially expressed genes, KCNQ genes were dramatically downregulated. KCNQ genes encode Kv7 channel proteins, which modulate neuronal excitability. Injection of both the Kv7.2/3 agonist ICA069673 and the Kv7.4 agonist fasudil into the VTA attenuated MA-induced conditioned place preference and locomotor sensitization and decreased neuronal excitability. Increasing Kv7.2/3 activity decreased neural oscillations, synaptic plasticity and DA release in the VTA-NacLat circuit in MA-treated mice. Furthermore, overexpression of only Kv7.3 channels in the VTA-NacLat circuit was sufficient to attenuate MA-induced reward behavior and decrease VTA neuron excitability. Activation of Kv7 channels in the VTA may become a novel treatment strategy for MA abuse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–98.
UNODC. United Nations Office on Drug and Crime (UNODC) United Nations. World Drug Report. 2022.
Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202.
Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78.
Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9.
Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8.
Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8.
Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114.
Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981;1:1361–8.
Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53.
Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–97.
Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci USA. 2006;103:2938–42.
Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–13.
Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73.
Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–62.
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–34.
Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–31.
Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–99.
Bassareo V, Musio P, Di, Chiara G. Reciprocal responsiveness of nucleus accumbens shell and core dopamine to food- and drug-conditioned stimuli. Psychopharmacology (Berl). 2011;214:687–97.
Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–8.
Bassareo V, De Luca MA, Di, Chiara G. Differential impact of pavlovian drug conditioned stimuli on In Vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl). 2007;191:689–703.
Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91.
Lipovsek M, Bardy C, Cadwell CR, Hadley K, Kobak D, Tripathy SJ. Patch-seq: past, present, and future. J Neurosci. 2021;41:937–46.
Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol. 2016;34:199–203.
Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–3.
Jensen HS, Grunnet M, Olesen SP. Inactivation as a new regulatory mechanism for neuronal Kv7 channels. Biophys J. 2007;92:2747–56.
Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda). 2011;26:365–76.
Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30.
Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23.
Brueggemann LI, Cribbs LL, Byron KL. Heteromeric channels formed from alternating Kv7.4 and Kv7.5 alpha-subunits display biophysical, regulatory, and pharmacological characteristics of smooth muscle M-currents. Front Physiol. 2020;11:992.
Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–46.
Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–95.
Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. J Biol Chem. 2000;275:13343–8.
Zaika O, Hernandez CC, Bal M, Tolstykh GP, Shapiro MS. Determinants within the turret and pore-loop domains of KCNQ3 K+ channels governing functional activity. Biophys J. 2008;95:5121–37.
Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–90.
Carver CM, Shapiro MS. Gq-coupled muscarinic receptor enhancement of KCNQ2/3 channels and activation of TRPC channels in multimodal control of excitability in dentate gyrus granule cells. J Neurosci. 2019;39:1566–87.
Varghese N, Lauritano A, Taglialatela M, Tzingounis AV. KCNQ3 is the principal target of retigabine in CA1 and subicular excitatory neurons. J Neurophysiol. 2021;125:1440–9.
Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27:1853–67.
Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–24.
Hansen HH, Ebbesen C, Mathiesen C, Weikop P, Ronn LC, Waroux O, et al. The KCNQ channel opener retigabine inhibits the activity of mesencephalic dopaminergic systems of the rat. J Pharmacol Exp Ther. 2006;318:1006–19.
Martire M, D’Amico M, Panza E, Miceli F, Viggiano D, Lavergata F, et al. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007;102:179–93.
Li L, Sun H, Ding J, Niu C, Su M, Zhang L, et al. Selective targeting of M-type potassium K(v) 7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour. Br J Pharmacol. 2017;174:4277–94.
Tan X, Liu X, Liu E, Liu M, Mu S, Hang Z, et al. Astrocyte-derived lactate/NADH alters methamphetamine-induced memory consolidation and retrieval by regulating neuronal synaptic plasticity in the dorsal hippocampus. Brain Struct Funct. 2022;227:2681–99.
Tackenberg C, Ghori A, Brandt R. Thin, stubby or mushroom: spine pathology in Alzheimer’s disease. Curr Alzheimer Res. 2009;6:261–8.
Wang Z, Jin T, Le Q, Liu C, Wang X, Wang F, et al. Retrieval-driven hippocampal NPTX2 plasticity facilitates the extinction of cocaine-associated context memory. Biol Psychiatry. 2020;87:979–91.
Devienne G, Le Gac B, Piquet J, Cauli B. Single cell multiplex reverse transcription polymerase chain reaction after patch-clamp. J Vis Exp. 2018;136:57627.
Xiao T, Wang Y, Wei H, Yu P, Jiang Y, Mao L. Electrochemical monitoring of propagative fluctuation of ascorbate in the live rat brain during spreading depolarization. Angew Chem Int Ed Engl. 2019;58:6616–9.
Liu X, Xiao T, Wu F, Shen MY, Zhang M, Yu HH, et al. Ultrathin cell-membrane-mimic phosphorylcholine polymer film coating enables large improvements for In Vivo electrochemical detection. Angew Chem Int Ed Engl. 2017;56:11802–6.
Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63.
Provence A, Malysz J, Petkov GV. The novel KV7.2/KV7.3 channel opener ICA-069673 reveals subtype-specific functional roles in guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther. 2015;354:290–301.
Zhang X, An H, Li J, Zhang Y, Liu Y, Jia Z, et al. Selective activation of vascular K(v) 7.4/K(v) 7.5 K(+) channels by fasudil contributes to its vasorelaxant effect. Br J Pharmacol. 2016;173:3480–91.
Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–95.
Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31:377–95.
Stefanics G, Hangya B, Hernadi I, Winkler I, Lakatos P, Ulbert I. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci. 2010;30:13578–85.
van Wingerden M, Vinck M, Lankelma J, Pennartz CM. Theta-band phase locking of orbitofrontal neurons during reward expectancy. J Neurosci. 2010;30:7078–87.
Kaplan R, Bush D, Bonnefond M, Bandettini PA, Barnes GR, Doeller CF, et al. Medial prefrontal theta phase coupling during spatial memory retrieval. Hippocampus. 2014;24:656–65.
O’Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. 2013;33:14211–24.
Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Good vibrations: cross-frequency coupling in the human nucleus accumbens during reward processing. J Cogn Neurosci. 2009;21:875–89.
Lega BC, Kahana MJ, Jaggi J, Baltuch GH, Zaghloul K. Neuronal and oscillatory activity during reward processing in the human ventral striatum. Neuroreport. 2011;22:795–800.
De Pascalis V, Varriale V, D’Antuono L. Event-related components of the punishment and reward sensitivity. Clin Neurophysiol. 2010;121:60–76.
Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–500.
Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34:1023–35.
Li Y, Pehrson AL, Waller JA, Dale E, Sanchez C, Gulinello M. A critical evaluation of the activity-regulated cytoskeleton-associated protein (Arc/Arg3.1)‘s putative role in regulating dendritic plasticity, cognitive processes, and mood in animal models of depression. Front Neurosci. 2015;9:279.
Lv XF, Xu Y, Han JS, Cui CL. Expression of activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) in the nucleus accumbens is critical for the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Behav Brain Res. 2011;223:182–91.
Mabb AM, Ehlers MD. Arc ubiquitination in synaptic plasticity. Semin Cell Dev Biol. 2018;77:10–6.
Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69.
Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9.
Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98.
Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl). 2007;191:461–82.
Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol. 2006;96:535–43.
Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels. Br J Pharmacol. 2000;129:413–5.
Zhao C, Su M, Wang Y, Li X, Zhang Y, Du X, et al. Selective Modulation of K(+) Channel Kv7.4 Significantly Affects the Excitability of DRN 5-HT Neurons. Front Cell Neurosci. 2017;11:405.
Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ, et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 2016;7:11671.
McNamara CG, Tejero-Cantero A, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–60.
Hipp JF, Hawellek DJ, Corbetta M, Siegel M, Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15:884–90.
Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504.
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58.
Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–2.
Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63.
Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–82.
Acknowledgements
We thank the Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Funding
This study was supported by the National Natural Science Foundation of China, Grant Number: U220011 and 81871044.
Author information
Authors and Affiliations
Contributions
JS and EL designed this experiment and prepared the manuscript. EL also performed experiments and wrote the draft. KP, ML, XT, RS, ZH, SM and WH participated in some of the experiments and analyzed the data. SC provided critical revision for important intellectual content and revised the manuscript draft. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, E., Pang, K., Liu, M. et al. Activation of Kv7 channels normalizes hyperactivity of the VTA-NAcLat circuit and attenuates methamphetamine-induced conditioned place preference and sensitization in mice. Mol Psychiatry 28, 5183–5194 (2023). https://doi.org/10.1038/s41380-023-02218-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02218-5