Abstract
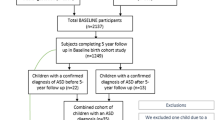
Epidemiological studies and work in animal models indicate that immune activation may be a risk factor for autism spectrum disorders (ASDs). We measured levels of 60 cytokines and growth factors in 869 maternal mid-gestational (MMG) and 807 child cord blood (CB) plasma samples from 457 ASD (385 boys, 72 girls) and 497 control children (418 boys, 79 girls) from the Norwegian Autism Birth Cohort. We analyzed associations first using sex-stratified unadjusted and adjusted logistic regression models, and then employed machine learning strategies (LASSO + interactions, Random Forests, XGBoost classifiers) with cross-validation and randomly sampled test set evaluation to assess the utility of immune signatures as ASD biomarkers. We found prominent case–control differences in both boys and girls with alterations in a wide range of analytes in MMG and CB plasma including but not limited to IL1RA, TNFα, Serpin E1, VCAM1, VEGFD, EGF, CSF1, and CSF2. MMG findings were most striking, with particularly strong effect sizes in girls. Models did not change appreciably upon adjustment for maternal conditions, medication use, or emotional distress ratings. Findings were corroborated using machine learning approaches, with area under the receiver operating characteristic curve values in the test sets ranging from 0.771 to 0.965. Our results are consistent with gestational immunopathology in ASD, may provide insights into sex-specific differences, and have the potential to lead to biomarkers for early diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5(R)). Arlington, VA: American Psychiatric Publishing; 2013.
Randall M, Egberts KJ, Samtani A, Scholten RJ, Hooft L, Livingstone N, et al. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev. 2018;7:CD009044.
Zwaigenbaum L, Penner M. Autism spectrum disorder: advances in diagnosis and evaluation. BMJ. 2018;361:k1674.
Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69:1–12.
Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26:146–53.
Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–703.
Wiggs KK, Rickert ME, Sujan AC, Quinn PD, Larsson H, Lichtenstein P, et al. Antiseizure medication use during pregnancy and risk of ASD and ADHD in children. Neurology. 2020;95:e3232–e3240.
Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86.
Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299:241–51.
Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2015;20:440–6.
Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–6.
Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. Prenatal fever and autism risk. Mol Psychiatry. 2018;23:759–66.
Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16:469–86.
Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–9.
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702.
Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78.
Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol Psychiatry. 2017;81:391–401.
Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–20.
Needleman LA, McAllister AK. The major histocompatibility complex and autism spectrum disorder. Dev Neurobiol. 2012;72:1288–301.
Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–45.
Carpentier PA, Dingman AL, Palmer TD. Placental TNF-alpha signaling in illness-induced complications of pregnancy. Am J Pathol. 2011;178:2802–10.
Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–57.
Azmitia EC, Saccomano ZT, Alzoobaee MF, Boldrini M, Whitaker-Azmitia PM. Persistent angiogenesis in the autism brain: an immunocytochemical study of postmortem cortex, brainstem and cerebellum. J Autism Dev Disord. 2016;46:1307–18.
Prins JR, Eskandar S, Eggen BJL, Scherjon SA. Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment? J Reprod Immunol. 2018;126:18–22.
Kurzrock R, Estrov Z, Wetzler M, Gutterman JU, Talpaz M. LIF: not just a leukemia inhibitory factor. Endocr Rev. 1991;12:208–17.
Probert L. TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. 2015;302:2–22.
Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252:75–82.
Abdallah MW, Larsen N, Grove J, Bonefeld-Jorgensen EC, Norgaard-Pedersen B, Hougaard DM, et al. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. 2013;61:370–6.
Abdallah MW, Mortensen EL, Greaves-Lord K, Larsen N, Bonefeld-Jorgensen EC, Norgaard-Pedersen B, et al. Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr Scand. 2013;128:61–69.
Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, et al. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2006;35:1146–50.
Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol. 2016;45:382–8.
Stoltenberg C, Schjolberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, et al. The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15:676–80.
Suren P, Havdahl A, Oyen AS, Schjolberg S, Reichborn-Kjennerud T, Magnus P, et al. Diagnosing autism spectrum disorder among children in Norway. Tidsskr Nor Laegeforen. 2019;139.
Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry. 2003;57:113–8.
Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–25.
Bach JF. Infections and autoimmune diseases. J Autoimmun. 2005;25:74–80.
Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol Autism. 2011;2:13.
Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–55.
Breen EJ, Tan W, Khan A. The statistical value of raw fluorescence signal in luminex xMAP based multiplex immunoassays. Sci Rep. 2016;6:26996.
Breen EJ, Polaskova V, Khan A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine. 2015;71:188–98.
Helsel DR. Fabricating data: how substituting values for nondetects can ruin results, and what can be done about it. Chemosphere. 2006;65:2434–9.
Antweiler RC. Evaluation of statistical treatments of left-censored environmental data using coincident uncensored data sets. II. Group comparisons. Environ Sci Technol. 2015;49:13439–46.
Aggarwal CC. Outlier analysis. 1st ed. New York: Springer-Verlag; 2013, pp. XV, 446.
Benjamini Y, Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57:289–300.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc: Ser B Methodol. 1996;58:267–88.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
Chen T, Guestrin C. XGBoost: a scalable tree boosting system. San Francisco, CA: 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; 2016, pp. 785–94.
Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Stat Sci. 1999;14:382–401.
Sanchez-Meca J, Marin-Martinez F, Chacon-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–67.
Cohen J. Statistical power anaysis for the behavioral sciences. 2nd edn. New York: Routledge; 1988.
Efron B, Tibshirani RJ. An introduction to the bootstrap. 1st ed. Norwell, Massachusetts: Chapman & Hall; 1993.
Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, et al. Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Res. 2017;10:1878–90.
Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43:25–33.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18.
Atzeni F, Sarzi-Puttini P. Tumor necrosis factor. In: Maloy S, Kelly H (eds). Brenner’s encyclopedia of genetics. 2nd ed. San Diego, CA: Academic Press; 2013, pp. 229–31.
Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72.
Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–5.
Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. 2015;5:e647.
Mizejewski GJ, Lindau-Shepard B, Pass KA. Newborn screening for autism: in search of candidate biomarkers. Biomark Med. 2013;7:247–60.
Mahic M, Mjaaland S, Bovelstad HM, Gunnes N, Susser E, Bresnahan M, et al. Maternal immunoreactivity to herpes simplex virus 2 and risk of autism spectrum disorder in male offspring. mSphere. 2017;2:e00016–17.
Mahic M, Che X, Susser E, Levin B, Reichborn-Kjennerud T, Magnus P, et al. Epidemiological and serological investigation into the role of gestational maternal influenza virus infection and autism spectrum disorders. mSphere. 2017;2:e00159–17.
Stolp HB. Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol Cell Neurosci. 2013;53:63–68.
Chitu V, Gokhan S, Nandi S, Mehler MF, Stanley ER. Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci. 2016;39:378–93.
Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–13.
Kana V, Desland FA, Casanova-Acebes M, Ayata P, Badimon A, Nabel E, et al. CSF-1 controls cerebellar microglia and is required for motor function and social interaction. J Exp Med. 2019;216:2265–81.
Zengeler KE, Lukens JR. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat Rev Immunol. 2021;21:454–68.
Han VX, Patel S, Jones HF, Dale RC. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat Rev Neurol. 2021;17:564–79.
Costa D, Castelo R. Umbilical cord gene expression reveals the molecular architecture of the fetal inflammatory response in extremely preterm newborns. Pediatr Res. 2016;79:473–81.
Shen L, Liu X, Zhang H, Lin J, Feng C, Iqbal J. Biomarkers in autism spectrum disorders: current progress. Clin Chim Acta. 2020;502:41–54.
Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–54.
Acknowledgements
We dedicate this paper to the memory of Sir Michael (Mike) Rutter, a dear friend and pioneer in autism and child psychiatry research, who helped build the ABC. We thank Wai Hung Wong, Nina Deoras, Parisa Zolfaghari, and Shobun Baile for laboratory analyses, Joy Ukaigwe for data preparation, Meredith Eddy for project coordination, and Kelly Magnus for assistance with manuscript preparation. We are grateful to the families in Norway participating in MoBa and the ABC study. This work was supported by National Institutes of Health grants NS047537 and NS086122, the Jane Botsford Johnson foundation, the Korein Foundation, the Simons Foundation Autism Research Initiative, the Norwegian Ministry of Health and Care Services, the Norwegian Ministry of Education and Research, and Research Council of Norway grants 189457, 190694, and 196452. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
CS, ES, MB, MH, PM, and WIL developed the experimental design. MH directed cytokine assays. XC directed statistical analyses. WIL and XC wrote the manuscript. CS, ES, MB, MH, PM, PS, SM, TR-K, and WIL contributed to the data analysis, edited, and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Che, X., Hornig, M., Bresnahan, M. et al. Maternal mid-gestational and child cord blood immune signatures are strongly associated with offspring risk of ASD. Mol Psychiatry 27, 1527–1541 (2022). https://doi.org/10.1038/s41380-021-01415-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01415-4
This article is cited by
-
Cohort-guided insights into gene–environment interactions in autism spectrum disorders
Nature Reviews Neurology (2023)
-
Metabolomic analysis of maternal mid-gestation plasma and cord blood in autism spectrum disorders
Molecular Psychiatry (2023)
-
Association between history of miscarriage and autism spectrum disorder
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Autism spectrum disorders linked to gestational immune activation
Nature Reviews Neurology (2022)