Abstract
Chromosome banding analysis (CBA) remains the standard-of-care for structural variant (SV) assessment in MDS. Optical genome mapping (OGM) is a novel, non-sequencing-based technique for high-resolution genome-wide SV profiling (SVP). We explored the clinical value of SVP by OGM in 101 consecutive, newly diagnosed MDS patients from a single-center, who underwent standard-of-care cytogenetic and targeted NGS studies. OGM detected 383 clinically significant, recurrent and novel SVs. Of these, 224 (51%) SVs, seen across 34% of patients, were cryptic by CBA (included rearrangements involving MECOM, NUP98::PRRX2, KMT2A partial tandem duplications among others). SVP decreased the proportion of normal karyotype by 16%, identified complex genomes (17%), chromothripsis (6%) and generated informative results in both patients with insufficient metaphases. Precise gene/exon-level mapping allowed assessment of clinically relevant biomarkers (TP53 allele status, KMT2A-PTD) without additional testing. SV data was complementary to NGS. When applied in retrospect, OGM results changed the comprehensive cytogenetic scoring system (CCSS) and R-IPSS risk-groups in 21% and 17% patients respectively with an improved prediction of prognosis. By multivariate analysis, CCSS by OGM only (not CBA), TP53 mutation and BM blasts independently predicted survival. This is the first and largest study reporting the value of combined SVP and NGS for MDS prognostication.
Similar content being viewed by others
Introduction
Myelodysplastic syndromes (MDS) are characterized by a high degree of somatic genetic alterations ranging from single-nucleotide mutations to intermediate and large-sized structural variants (SVs) [1,2,3,4]. SVs are defined as genomic alterations greater than 50 base pairs (bp) that encompass copy number variants (CNVs: deletions, and duplications), insertions, inversions, gene rearrangements, complex genomic alterations, and copy-neutral loss-of-heterozygosity (CN-LOH)/uniparental disomy [5, 6]. Identification of SVs, largely evaluated by conventional chromosome banding analysis (CBA), is critical for diagnosis, prognostic risk-stratification of MDS and therapeutic decision making [3, 4, 7,8,9,10,11,12,13,14,15]. Recent data in MDS, using TP53 as a prototype, has highlighted the importance of assessment of both somatic mutations and SVs (deletions, CN-LOH, rearrangements) in genomic regions of interest for accurate prognostication [16, 17].
While the mutational landscape of MDS has been extensively characterized using next-generation sequencing (NGS), there is little information regarding unbiased high-resolution structural variant profiling (SVP) [2]. This is due to the limited ability of NGS to align and assemble segments from repetitive sequences, which constitute ~50% of human genome and harbor most of the complex SVs [18,19,20,21]. Therefore, to date, CBA remains the gold standard in the evaluation of MDS. However, CBA has limited resolution and poor sensitivity in detection of some clinically actionable alterations, and on occasions, can be non-informative due to the requirement of cell culture. In these contexts, and in the setting of failed or insufficient metaphases, as recommended by NCCN, these findings need to be supplemented with additional assay(s) such as fluorescence in situ hybridization (FISH) and chromosomal microarray (CMA), resulting in increasing costs and turn-around-times for the laboratory [22,23,24]. As a result, the knowledge of the full spectrum and prevalence of genome-wide high-resolution chromosomal SVs in MDS remains limited.
Optical genome mapping (OGM) is a novel, non-sequencing-based technique that enables detection of all SVs by evaluation of patterns generated by fluorophore tags labeled to specific 6 bp sequence motifs within extremely long DNA molecules (>300 kb), and is, therefore, unaffected by repeat sequences. The ability to detect all types of SVs in a single assay enabling unbiased structural variant profiling (SVP) at a high-resolution has been shown in constitutional and prenatal disorders, solid tumors, and a few hematological malignancies [25,26,27,28,29,30]. OGM lacks base-level resolution, which can be overcome by simultaneous targeted NGS-based mutational analysis, facilitating comprehensive evaluation of genomic landscape.
In this study, we performed pan-genomic profiling of a large cohort of newly diagnosed MDS patients and evaluated the impact on clinical prognostication using a combination of high-resolution SVP by OGM and mutation profiling by targeted deep NGS. The goal of this study was to identify the application of routine SVP in MDS by defining the spectrum of high-resolution SVs by OGM and evaluating the clinical impact of the information by clinicopathologic and outcome correlation.
Materials and methods
Sample selection
We used bone marrow (BM) aspirates from consecutive treatment naïve MDS patients who presented to our institution with adequate fresh/ frozen material available for OGM. The study was approved by the Institutional Review Board, all samples were collected following institutional guidelines with informed consent in accordance with the Declaration of Helsinki. Risk-stratification was performed using comprehensive cytogenetic scoring system (CCSS) and IPSS-R [3, 4].
Cytogenetic and molecular testing
All patients underwent BM aspiration and biopsy for diagnosis per 2016 WHO criteria [7] and standard-of-care testing in CLIA-certified laboratories that included targeted 81-gene NGS panel (Supplementary Table S1) [31,32,33,34,35,36], CBA by traditional G-banding and FISH/CMA (as needed) per standard protocols described elsewhere [37,38,39,40]. Additional FISH or CMA testing was performed to confirm most of the clinically significant OGM findings that were cryptic by CBA. All details are in Supplementary Material.
Optical genome mapping (OGM)
DNA extraction, labeling, and chip loading
We used BM mononuclear cells (BMMNCs) isolated from patient samples by ficoll density-gradient centrifugation that were either stored as viable cells in liquid nitrogen or cell pellets at −80 °C within 3 days of collection. Ultra-high molecular weight (UHMW) genomic DNA (gDNA) was extracted from 1.5 million cells following manufacturer’s protocols (Bionano Prep SP Blood and Cell DNA Isolation Kit; Bionano Genomics, San Diego, CA). Briefly, after thawing in 37 °C water bath, cryopreserved BMMNCs were washed with DNA stabilizing buffer to get the cell pellet, which was re-suspended in 40 ul DNA stabilizing buffer, lysed and digested with proteinase K, RNase A and buffer LBB. After PMSF treatment (Sigma-Aldrich, St. Louis, MO), nanobind paramagnetic disk was added to the solution, mixed with isopropanol to precipitate the gDNA and washed with WB1 and WB2. After transferring to a new tube, buffer EB (Bionano Genomics, San Diego, CA) was added to elute the gDNA from the disk. The gDNA was equilibrated overnight at room temperature for homogenization and subsequently quantified with Qubit Fluorometer (Qubit BR dsDNA assay kit; ThermoFisher Scientific, CA).
Sequence-specific Direct label and stain (DLS) technique was used for labeling following manufacturer’s protocols (Bionano Prep DLS Labeling Kit; Bionano Genomics, San Diego, CA). Direct Labeling Enzyme 1 (DLE-1) reaction was carried out using 750 ng of gDNA to tag a specific 6 bp sequence (CTTAAG) with a DL-green fluorophore (~15 times per 100 kb). Following puregene proteinase K (Qiagen, Hilden, Germany) digestion and DL-Green clean-up using DLS membrane in DLS 24-well plate, the labeled gDNA was mixed with DNA stain, stained overnight at room temperature for backbone visualization and quantified using Qubit HS dsDNA assay kit (ThermoFisher Scientific, CA). The fluorescent-labeled gDNA molecules were loaded on a Saphyr chip G2.3, and linear double stranded molecules passing across nanochannels were sequentially imaged on a Saphyr instrument (Bionano Genomics, San Diego, CA) (Supplementary Fig. S1).
Data analysis and variant filtering
Effective genome coverage of approximately 300X was achieved for every tested sample (1300 GB data per sample), theoretically enabling detection of aberrations at a 5% allele frequency (equivalent to 10% of cells when heterozygous). Standard run quality control parameters [total DNA ( ≥150 kbp), N50 ( ≥150 kbp), map rate (≥150 kbp), effective coverage (>300x), average label density (per 100 kbp)] were evaluated per manufacturer’s guidelines. Details regarding limit of detection, reproducibility and precision are described in Supplementary Material [41, 42].
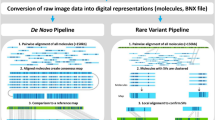
Data was analyzed using Bionano Access (Bionano Genomics, San Diego, CA) using Genome Reference Consortium GRCh38/hg38 as the reference. Data analysis was performed in a single-blinded fashion independently by 2 users using de novo (DN; SVs > 500 bp), rare variant (RV; SVs > 5000 bp) and copy number pipelines (CN; capturing large CNVs > 500,000 bp potentially missed by SV algorithms). RV pipeline enabled detection of SVs occurring at low allelic fractions (~10%), as determined by prior sensitivity studies using simulations and serial dilutions of cell lines (data not shown). DN pipeline was primarily used for CN-LOH assessment and confirmation of SV calls > 5000 bp detected by RV pipeline; SV calls between 500 bp and 5000 bp were not included for this study.
For variant filtering, first, we used the recommended size and confidence score filters for each of the three pipelines for to generate a list of high confidence SVs and copy number variants for analysis described elsewhere (Supplementary Table S2) [27, 28, 30, 41]. Second, we used the OGM data generated from 200 healthy individuals to select only rare variants that represent pathogenic somatic alterations. As a final third step, in order to select SVs that are of clinical significance, we selected SVs that overlapped the coding region of a gene/ chromosome locus implicated in myeloid neoplasm (Supplementary Table S3), adapted from the publicly available myeloid neoplasm-specific gene list [Cancer Genomics Consortium (https://www.cancergenomics.org/gene_lists.php)] and in-house 81-gene NGS panel. The final interpretation of every call was made after visualizing the changes in the sequence patterns in the molecules from sample compared to the reference.
Statistical analysis
Overall survival (OS) was calculated from the time from diagnosis to death or the last follow-up date. Patients who were alive at their last follow-up were censored on that date. The Kaplan–Meier product limit method was used to estimate the median OS for each parameter. Univariate Cox proportional hazards regression analysis was used to identify association of each of the variables with OS, followed by multivariate analysis (using p = 0.2 cut-off to select variables). Prognostic fitness of cytogenetic risk calculated from CBA and OGM were assessed using Harrell’s concordance index.
Results
Baseline patient characteristics
The study cohort included 101 consecutive patients with newly diagnosed and treatment naïve MDS. Baseline patient characteristics are shown in Table 1. A total of 62 (62%) patients had at least 1 clonal cytogenetic alteration, with a median of 1 (0–12) abnormality per patient. Two patients had insufficient metaphases. The median number of somatic gene mutations was 2 (0–7) per patient. The most frequent mutations (seen in >5% of patients) were TET2 (31%), SF3B1 (28%), TP53 (22%), ASXL1 (17%), RUNX1 (12%), SRSF2 (9%), DNMT3A (7%), and IDH1/2 (5%). The distribution of the cytogenetic risk by CCSS, R-IPPS risk categories and somatic mutations were representative of a real-world setting, with a slightly increased frequency of complex karyotype (CK, 29%) and/or TP53 mutations (22%) due to tertiary care referral bias.
SVP of MDS using high-resolution OGM
Using a single platform for unbiased SV detection enabled systematic detection of all types of somatic SVs at the same resolution without the need for germline DNA. A median coverage of >300x was achieved on all samples, permitting a detection sensitivity of ~10%. There were 4030 unique somatic variant calls, of which 383 (9.5%; included 222 CNVs; 161 SVs) were classified as “clinically significant” based on the overlap with a gene or genomic region of interest (Fig. 1). These included a broad spectrum of SV types involving both recurrent and novel regions/ genes of interest: CN aberrations (gains/losses), inversions, balanced and unbalanced rearrangements (intrachromosomal and interchromosomal, including three-way translocations). Specific patterns of chromosomal alterations such as dicentric and ring chromosomes, and isochromosomes were readily apparent. Visualization of multiple aberrations per sample enabled reconstruction of complex derivative chromosomes, and complex alterations of chromoanagenesis (chromothripsis, chromoplexy and chromoanasynthesis). In addition to gene-level alterations, structural alterations at the exon-level such as partial tandem duplications involving KMT2A (KMT2A-PTD) were detected. Copy-neutral loss-of-heterozygosity (CN-LOH), evaluated in a subset (n = 70) of patients frequently involved chromosome 11 followed by chromosomes 12 and 13. In total, OGM identified at least 1 clinically significant SV in 70 of 101 MDS patients (70%, compared to 62% by CBA), with a median of 1 (range, 0–47) per patient. Supplementary Fig. S2 shows representative examples SVs of different types.
A A total of 383 of 4030 unique copy number variant (CNV) (both gains and losses) plus SV calls (with subtypes, included partial tandem duplications, all involving KMT2A on chromosome 11, inversions, intra-chromosomal and inter-chromosomal translocations), as well as copy neutral loss-of-heterozygosity (CN-LOH) calls were identified. B–D The distribution of CNVs (B), SVs (C) and CN-LOH (D) represented on the Y-axes plotted across different chromosomes (chromosome number on the X-axis).
Comparison of SVs between OGM and chromosome banding analysis (CBA)
The distribution of chromosomal aberrations (CNVs, SVs and CN-LOH) detected by OGM compared to standard-of-care CBA is shown in Fig. 2. Even after excluding CN-LOH that cannot be detected by CBA, less than half (n = 189, 49%) of clinically significant SVs identified by OGM were detected by CBA. The remaining 224 (51%) clinically significant SVs, detected across 34 (34%) patients were cytogenetically silent, and only became apparent by OGM, either due to the sub-microscopic (below the limit of resolution) or cryptic nature of the alterations, or poor chromosomal morphology associated with CK. These included alterations such as segmental deletions in chromosomes 5q and 7q, TP53 deletions and rearrangements, and KMT2A deletions, amplifications, and PTDs. By OGM, the proportion of MDS patients with normal karyotype (NK) decreased by 16% compared to CBA (not considering CN-LOH). Within the setting of NK, OGM uncovered cryptic rearrangements involving MECOM, NUP98::PRRX2 (1 patient), KMT2A-PTD (in 2 patients), and deletions of genomic regions encompassing TET2, KMT2A, and ETV6 (in 2 patients). Among those with CK, 17 (59%) patients not only demonstrated additional number of alterations, but a higher degree of genomic complexity than was apparent by CBA, and six (6%) patients showed chromoanagenesis (Supplementary Fig. S3). The higher resolution permitted clarification of uncertainties such as markers and additional material of unknown origin. In both patients with incomplete cytogenetic results, genome mapping generated successful sequence patterns (both showing NK) with adequate coverage and high-quality metric scores.
A, B OGM detected nearly twice the number of clinically significant structural variants (SVs); circos plots illustrating SVs by CBA (A) versus OGM (B). C 189 of 383 clinically significant aberrations, included SVs and copy number variants (CNVs), were cryptic; in addition, a subset of patients had copy-neutral loss-of-heterozygosity (CN-LOH).
Conversely, in 96 of 99 patients, all clonal cytogenetic abnormalities detected by CBA and could be confirmed by FISH/CMA were also evident by OGM. The missed aberrations included the following, all noted in 2-3 metaphases (a) +8 seen in 3 of 20 metaphases [possibly due to proliferation bias during cell growth, since FISH demonstrated +8 in ~1.6% of cells, well below the detection threshold of OGM), (b) t(9;10)(q32;q24) and, (c) del(9)(q13q22).
Integration of OGM and targeted NGS studies
By targeted NGS alone, 13 (13%) patients had no somatic mutation. Among these, OGM identified three patients with NUP98::PRRX2 and MECOM rearrangements. Among the rest, additional information of value included detection of KMT2A deletions in three additional patients and ETV6 deletion in one patient. Integrating data from both NGS and OGM detected at least 1 driver alteration in 97 of 101 patients. Specifically, among 5 patients with no cytogenetic aberrations or mutations by CBA and targeted NGS, OGM detected a genetic abnormality in 1 patient. Combined mutation and SV profiling correctly identified biallelic TP53 alterations in 3 patients (including a patient with wild-type TP53 by NGS, further details below), and notable associations, such as double RAS/FLT3 mutations in 2 of 3 KMT2A-PTD patients. The integrated findings of OGM, CBA and somatic mutations from each patient are shown in Fig. 3. Clinically significant SVs that were cryptic by CBA and detected by OGM alone were seen across all clinical, cytogenetic and mutational subsets, supporting the need for routine SVP in all MDS patients. The highest yield was seen in patients with higher number of cytogenetic abnormalities [CCSS score 3 (50%); 4 (90%)], R-IPSS [high (59%), very-high (70%)], and CK (~76%) [kappa: 0.776; SE = 0.049; p < 0.001]. Cryptic SVs were only identified in patients with ≤3 mutations detected by NGS, with the highest yield in patients with fewer mutations [1–2 per sample, ~48%; MDS with TP53 (16, 73%) and SF3B1 mutations (7, 25%)] (Supplementary Fig. S4).
The heatmap shows two clusters of patients: left panel represents patients with clinically significant cryptic variants detected by OGM, and right panel represents patients without clinically significant findings by OGM, compared to CBA. Patients are sorted left to right starting from the highest number of cryptic variants detected by OGM alone, followed by total OGM alterations, findings in key genes informative for MDS, CBA findings, including those with uncertainties in the karyotype such as markers and additional material of unknown significance. The resulting changes to the CCSS and R-IPSS risk categories are depicted by an asterisk. The rest of the heatmap shows the corresponding gene mutation data from a concomitant 81-gene NGS panel. Legend is provided at the bottom.
SVs involving clinically informative biomarkers of MDS
Precise mapping of SVs at gene-level, and in certain genes at the exon-level, allowed assessment of clinically informative biomarkers, without the need for confirmatory assays. In the context of MDS, we identified these as TP53 for allele state, MECOMr, NUP98r, and KMT2A alterations. Of 22% TP53 mutated patients, 16% had a single TP53 mutation necessitating FISH and/or CMA testing to identify concomitant deletion and CN-LOH, respectively. OGM was able to correctly map concurrent TP53 gene deletions in 2 of these patients, confirming biallelic TP53 alterations. None had CN-LOH. In addition, one patient with wild-type TP53 showed concomitant TP53 deletion and translocation, both disrupting the gene (biallelic TP53 alteration). There were patients with NUP98 fusions (n = 2; 1 cryptic by CBA) and MECOM alterations (n = 5; 4/5 cryptic). Other alterations that are less well-defined with respect to clinical prognosis included deletions of TET2 (n = 3; 2 cryptic) and ETV6 (7; 2 cryptic). Within KMT2A, a multitude of structural alterations affecting the genomic regions of interest were detectable using a single platform. These included fusions (n = 1), deletions (n = 9; 2 cryptic), gains (n = 1) and partial tandem duplications (n = 3, all cryptic) involving exons 3 to 6, thereby expanding the scope of genetic analysis (Table 2; Fig. 4). The findings were confirmed using orthogonal assays.
These included MDS patients with normal karyotype showing (A) partial tandem duplications affecting KMT2A, and (B) MECOM rearrangements of MECOM. MDS patient with wild-type TP53 by NGS showing biallelic TP53 alterations, deletion and translocation, both disrupting the gene (C) and MDS patient with normal karyotype showing cryptic NUP98::PRRX2 as shown in the circos plot comprising chromosomes 9 and 11 (D) and within the genome browser (E).
OGM enabled detailed molecular characterization of rare SVs, such as identification of ELL as the gene partner for KMT2A in a case of WT1 mutated MDS with t(11;19)(q23;p13.1). Direct visualization of molecules of t(9;11)(p23;q22) in an SF3B1 mutated MDS revealed a rearrangement involving SYTL2 gene along with a deletion of KMT2A (confirmed by FISH and chromosomal mapback). In another SF3B1/RUNX1 mutated MDS, t(1;3)(p36;q21) could be mapped to PRDM16::RPN1.
Changes in IPSS-R and clinical impact based on OGM results
To analyze the potential clinical impact of this information, we calculated and compared CCSS groups and R-IPSS categories by both techniques retrospectively. In the case of OGM, we recalculated the CCSS categories by alignment of multiple individual SVs to generate a predicted karyotype resulting in 224 unique SVs, with 94 (40%) being cryptic, detected across 34 patients. This upgraded the CCSS cytogenetic-risk in 12 patients (resulting in upgrade of R-IPSS in 9 patients) and downgraded in 7 patients (with downgrading of R-IPSS in 6 patients). Together with the generation of results for 2 patients with non-informative karyotype, OGM derived results led to modification of the cytogenetic-risk for 21% patients, leading to an overall change in R-IPSS risk category in 17 (17%) patients (Supplementary Table S4; Fig. 5).
A Flow plots showing modifications to the CCSS (comprehensive cytogenetic scoring system) in 21% of patients and R-IPSS (revised international prognostic scoring system) in 17% of patients based on OGM results of the MDS study cohort. B Kaplan–Meier curves showing the overall survival of MDS patients stratified by CCSS cytogenetic-risk categories evaluated from CBA versus OGM results (OGM had a slightly improved prediction rate based on a slightly higher c-index).
CCSS change was primarily due to the change in the number of cytogenetic abnormalities, although the type of aberration and their combination also affected some cases. OGM upgraded CCSS scores in 12 MDS patients by uncovering one or more cryptic SV(s) that increased the overall number of cytogenetic abnormalities. This affected cases with NK, non-complex or complex karyotype with 3 abnormalities and resulted in CCSS upgrade by either 1 (in 9 patients) or 2 points (in 3 patients). More interestingly, OGM data downgraded the CCSS scores in 7 patients. In 5 patients, this was due to the higher resolution of OGM leading to clarification of karyotype in the setting of markers, additional material, and derivative chromosomes, which ultimately resulted in decrease in the overall number of cytogenetic abnormalities. In 2 patients, this was due to failure of OGM to detect low-level aberrations, and in 1 patient, a cryptic del(11q) in a NK MDS patient downgraded the CCSS from “good” to “very good”.
To capture the clinical impact of SVs that do not always change the CCSS/R-IPSS, we used an alternate approach. We defined an “actionable SV” as (1) prognostic, not recognized in the CCSS criteria (KMT2A-PTD, TP53 alterations, knowledge essential to determine allele status) or (2) potential eligibility for an ongoing clinical trial (at the time of manuscript submission) such as KMT2Ar, MECOMr, or NUP98r. Well-established SVs defined in the CCSS were ignored, since these were already evaluated in the first approach. Based on this definition, 13 (13%) patients had an “actionable alteration” (9 with a positive finding) (Supplementary Table S5). When both CCSS change and actionable alterations were combined, OGM results could have informed data for prognosis/therapy in 28 (28%) patients.
Treatment information was available in 99 patients, of which 36 (36%) received disease modifying therapy for MDS [included hypomethylating agent (HMA) only (36%), HMA with investigational agents (50%), HMA with chemotherapy (6%) and chemotherapy only in 3 (8%) patients]. The remaining (64%) patients did not receive therapy (Table 1). Over a median follow-up of 34 months, the median overall survival was 86 months. Based on a higher concordance index, OGM had a slightly improved prediction of prognosis for both CCSS [0.7 (SE = 0.052) vs. 0.665 (SE = 0.053)] and R-IPSS [0.705 (SE = 0.046) vs. 0.69 (SE = 0.05)] compared to CBA, but the differences were not statistically significant. By univariate analysis, the following parameters associated with outcome: SF3B1 mutation [p = 0.030; HR 0.343; CI: 0.130–0.901], TP53 mutation [p = 0.001; HR 3.232; CI: 1.582–6.601], CCSS score by CBA [p = 0.001; HR 1.690; CI: 1.250–2.286] and CCSS by OGM [p < 0.001; HR 2.051; CI: 1.488–2.827]. By multivariable analysis, CCSS by OGM [p < 0.001; HR 1.956; CI: 1.367–2.798], but not CCSS by CBA), BM blasts [p = 0.026; HR 1.079; CI: 1.009–1.153] and TP53 mutation [p = 0.040; HR 2.335; CI: 1.038–5.250] associated with prognosis (Table 3).
Discussion
In this study, we used OGM and targeted NGS to explore a spectrum of high-resolution (≥5000 bp) clinically significant SVs in MDS that escaped detection by standard-of-care CBA and targeted NGS designed for mutation analysis. The findings showed the lack of the current standard-of-care workflow to comprehensively evaluate the genomic landscape of MDS and provided insight into a plethora of SV data that could be potentially investigated for developing targeted therapeutics. Importantly, OGM data was complementary to NGS data.
Recent whole genome and whole transcriptomic sequencing studies have demonstrated the feasibility in detecting targeted SVs, defined in European Leukemia Network (ELN) risk-stratification for AML [43] and fusions associated with ALL [44]. However, unbiased whole genome SV detection remains a challenge, particularly within the setting of complex MDS genomes, due to the inherent technical limitations of sequencing. Short-read NGS, routinely used for mutation analysis, has a low sensitivity for detecting SVs due to challenges in the alignment of repetitive sequences that are hot-spots for SVs [18,19,20]. Current long-read (10–20 kbp) sequencing technologies have improved alignment but are prone for high systematic error rates [18, 21]. These problems could potentially be overcome using non-sequencing based OGM that allows for detection of all types of SVs by evaluating patterns generated by fluorophore tags labeled to sequence-specific motifs in ultra-long DNA molecules. Together with targeted deep NGS, this approach could provide high-resolution genomic data for precision therapeutics.
For this exploratory study, we selected MDS as a prototype due to the marked cytogenomic heterogeneity, immense clinical need for accurate genomic prognostication and expansion of therapeutic targets. Despite a stringent definition, we identified several cryptic SVs of prognostic and therapeutic potential that were not readily apparent by standard evaluation in 34% of patients. This information changed the CCSS score in 21% and R-IPSS risk in 17% (1 in ~7) patients. CCSS scoring by OGM independently associated with survival by multivariable analysis. Since CCSS and IPSS-R were developed in untreated patients at diagnosis and during the disease course, we explored the effect of therapy. By univariate analysis, therapy only slightly improved the survival (p-value 0.586, HR: 0.819), and did not reach statistical significance. Including therapy in multivariable analysis did not change the results. One likely interpretation for these data is that the number of patients is limited, and therefore these results are not a reflection on the efficacy of therapy.
In addition, OGM uncovered “actionable alterations” in 13 (13%) of patients not apparent by CBA, that did not necessarily change CCSS/R-IPSS but qualified patients for specific therapeutic strategies of potential benefit [UPN90: cryptic MECOM rearrangement; UPN51: biallelic TP53 inactivation from a concurrent rearrangement and deletion, in the absence of a TP53 mutation; both patients had no change to their CCSS] [45, 46].
Beyond CNVs that are frequent in MDS, the study revealed cryptic SVs potentially amenable to targeted therapeutics. For example, leukemias with MECOMr, NUP98r, KMT2Ar, and KMT2A-PTD are being explored as therapeutic targets within clinical trials [NCT04067336; NCT04811560] [10,11,12,13,14,15]. KMT2A-PTD is independently associated with poor prognosis and included in the IPSS-M model under development by IWG-PM. Due to the need for long-range PCR assays and difficultly in detection by NGS, limited laboratories perform KMT2A-PTD assessment. The frequency of KMT2A-PTD in our cohort (~3%) is similar to other reports [16, 47,48,49]. The finding of exon-level details suggests that OGM may bridge the gap between the large chromosomal alterations observed by CBA and small insertions/ deletions detectable by routine short-read NGS, which remain largely uninterrogated in routine work-up. This also emphasizes the complementary nature of OGM and NGS data, and co-operative interplay between SVs and mutations involving leukemogenic genes, as has been shown for TP53.
Finally, CN-LOH information can be extracted from OGM data within de novo assembly pipeline. Genomic regions of CN-LOH show consistent decrease in heterozygous SV calls compared to the level observed genome-wide in controls. CN-LOH can be easily distinguished from LOH resulting from a gain or a loss by reviewing the copy number changes using whole genome and circos plots. Although not included in the IPSS-R for calculation of the number of cytogenetic abnormalities, CN-LOH, specifically involving TP53 gene is necessary for allele state determination and MDS prognostication using the latest IPSS-M [49].
The results of the study demonstrate that we are grossly under evaluating the degree of genomic aberrations using standard-of-care techniques. On top of significant increment in the diagnostic yield, OGM detected different types of SVs using one platform, rather than a multitude of techniques with different detection sensitivities and shortcomings. For example, CMA provides data on high-resolution CNA(s) and CN-LOH but lacks the ability to identify balanced translocations and inversions and resolve complex SVs [22]. With the emerging prognostic models that incorporate granular cytogenomic data (TP53 allele state, KMT2A-PTD), a single platform that enables comprehensive detection of all types of chromosomal alterations becomes invaluable. The ability to consolidate multiple clinical assays could also simplify the laboratory workflow, and lead to better utilization of technical expertise and resources. The average duration between sample receipt and interpretation of results was ~5 days (3–4-day run-time; 1 day for analysis). OGM can be readily implemented for comprehensive cytogenetic analysis without sophisticated bioinformatic support but would need a separate targeted NGS for single nucleotide level mutation analysis. Recent data showed WGS (60X coverage) as a feasible alternative to CBA, with simultaneous mutation data, both of which improved the risk-stratification of a subset of intermediate-risk AML [43]. When compared to targeted NGS (median ~1500X), mutation yield was compromised due to decreased depth of coverage, specifically in variants ≤35% VAF [50]. Considering those actionable mutations such as IDH1/2 and FLT3, are often sub-clonal, and the current eligibility criteria for clinical trials is based on the presence or absence of mutations rather than the VAFs, this may pose specific challenges in enrollment for these patients [50,51,52]. Further, unbiased interpretation of all mutational variants by WGS, beyond those pertinent to ELN risk, will need simultaneous profiling of matched germline tissue [53].
The current study still underestimates the diagnostic yield of OGM and the clinical significance. For the sake of high stringency, we did not evaluate SVs between 500–5000 bp although detectable by de novo pipeline, SVs involving non-coding regions and those affecting genomic regions not implicated in myeloid cancers. R-IPSS model has not evaluated the clinical importance of some adverse prognostic SVs other than MECOMr, such as NUP98r, KMT2Ar, KMT2A-PTDs, chromoanagenesis due to either rarity or limited scope of traditional techniques. These need to be revisited in the future, within the context of mutations, using emerging technologies.
This is the first study to evaluate the clinical importance of SVP in the largest single center MDS cohort. However, there are some limitations. The study design is retrospective with an initial goal to explore the feasibility and clinical value of SVP. The findings need independent validation, on a large-scale in prospective multi-center cohorts within clinical trials. Unlike CBA, OGM cannot provide data at single-cell level (similar to all bulk nucleic-acid assays), which limits understanding of sub-clonal architecture. Nonetheless, this study presents evidence and opportunities for furthering efforts to facilitate a more precise genomic classification beyond current knowledge. Accumulating evidence from multiple such studies will guide their graded implementation into clinical laboratories and eventually set a new norm for routine high-resolution SVP [24].
Along similar lines, direct assessment of true biallelic inactivation of TP53 requires analysis of mutation(s), CNV(s), SV(s) and CN-LOH at a single-cell resolution, which is not feasible using any of the routine bulk sequencing methodologies (OGM, targeting NGS, WES, WGS). This information needs to be inferred from quantitative variant fractions [16], and the “deleterious” nature of the rarer mutation(s) substantiated by computational algorithms or functional evidence [34]. Nevertheless, the superior ability of OGM in SV characterization offers advantages when combined with either targeted, whole genome or exome sequencing, especially within tumor suppressor genes. Within the current study, we identified a case of MDS with TP53 biallelic inactivation by two different SVs (deletion and rearrangement), in the absence of a mutation. In another study, OGM outperformed WGS/WES in SV detection within repetitive and difficult to map genomic regions such as 22q11.2 due to the use of ultra-long DNA molecules [54]. OGM also enabled detection of unique types of SVs that can be missed by WES/WGS. In a separate study, OGM identified an intronic insertion (retrotransposon element) within the SMARCB1 tumor suppressor gene as the genetic cause of a congenital atypical rhabdoid tumor. This insertion was missed by CMA, WES, and WGS [55]. Therefore, it is important to be cognizant of the complementary nature of different genomic technologies [56].
In summary, our data provide support that pan-genomic profiling by combined OGM and targeted deep NGS, improves risk-stratification in MDS by uncovering a gamut of high-resolution actionable genomic aberrations.
Data availability
The datasets generated during and/or analyzed during this study are not publicly available due to patient privacy concerns but are available from the corresponding author upon request.
References
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506.
Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–27.
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65.
Schanz J, Tuchler H, Sole F, Mallo M, Luno E, Cervera J, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol. 2012;30:820–9.
Alkan C, Coe BP, Eichler EE. Genome structural variation discovery and genotyping. Nat Rev Genet. 2011;12:363–76.
Mahmoud M, Gobet N, Cruz-Davalos DI, Mounier N, Dessimoz C, Sedlazeck FJ. Structural variant calling: the long and the short of it. Genome Biol. 2019;20:246.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Tiu RV, Gondek LP, O’Keefe CL, Elson P, Huh J, Mohamedali A, et al. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011;117:4552–60.
Garcia-Manero G, Chien KS, Montalban-Bravo G. Myelodysplastic syndromes: 2021 update on diagnosis, risk stratification and management. Am J Hematol. 2020;95:1399–420.
Kura Oncology I. First in human study of KO-539 in relapsed or refractory acute myeloid leukemia. 2022 [cited; Available from: https://clinicaltrials.gov/ct2/show/NCT04067336.
Janssen Research & Development L. A Study of JNJ-75276617 in Participants With Acute Leukemia. 2022 [cited 2022; Available from: https://clinicaltrials.gov/ct2/show/NCT04811560.
Center FHCR. Donor peripheral blood stem cell transplant in treating patients with hematologic malignancies 2022 [cited; Available from: https://clinicaltrials.gov/ct2/show/NCT01028716.
Epizyme I A Phase 1, Open-Label, Dose-Escalation & Expanded Cohort, Continuous IV Infusion, Multi-center Study of the Safety, Tolerability,PK & PD of EPZ-5676 in Treatment Relapsed/Refractory Patients With Leukemias Involving Translocation of the MLL Gene at 11q23 or Advanced Hematologic Malignancies. 2022 [cited; Available from: https://www.clinicaltrials.gov/ct2/show/NCT01684150.
Stein EM, Garcia-Manero G, Rizzieri DA, Tibes R, Berdeja JG, Savona MR, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood. 2018;131:2661–9.
Hospital SJCsR. A trial of epigenetic priming in patients with newly diagnosed acute myeloid leukemia. 2022 [cited; Available from: https://clinicaltrials.gov/ct2/show/NCT03164057.
Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56.
Montalban-Bravo G, Kanagal-Shamanna R, Benton CB, Class CA, Chien KS, Sasaki K, et al. Genomic context and TP53 allele frequency define clinical outcomes in TP53-mutated myelodysplastic syndromes. Blood Adv. 2020;4:482–95.
Mantere T, Kersten S, Hoischen A. Long-read sequencing emerging in medical genetics. Front Genet. 2019;10:426.
Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet. 2011;13:36–46.
Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002;3:370–9.
Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17:333–51.
Kanagal-Shamanna R, Hodge JC, Tucker T, Shetty S, Yenamandra A, Dixon-McIver A, et al. Assessing copy number aberrations and copy neutral loss of heterozygosity across the genome as best practice: An evidence based review of clinical utility from the cancer genomics consortium (CGC) working group for myelodysplastic syndrome, myelodysplastic/myeloproliferative and myeloproliferative neoplasms. Cancer. Genetics. 2018;228:197–217.
National Cancer Comprehensive Network N. National Cancer Comprehensive Network (NCCN) Guidelines Version 3.2022. 2022 [cited; Available from: https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf.
Akkari YMN, Baughn LB, Dubuc AM, Smith AC, Mallo M, Dal Cin P, et al. Guiding the global evolution of cytogenetic testing for hematologic malignancies. Blood. 2022;139:2273–84.
Lestringant V, Duployez N, Penther D, Luquet I, Derrieux C, Lutun A, et al. Optical genome mapping, a promising alternative to gold standard cytogenetic approaches in a series of acute lymphoblastic leukemias. Genes Chromosomes Cancer. 2021;60:657–67.
Luhmann JL, Stelter M, Wolter M, Kater J, Lentes J, Bergmann AK, et al. The clinical utility of optical genome mapping for the assessment of genomic aberrations in acute lymphoblastic leukemia. Cancers (Basel). 2021;13:4388.
Mantere T, Neveling K, Pebrel-Richard C, Benoist M, van der Zande G, Kater-Baats E, et al. Optical genome mapping enables constitutional chromosomal aberration detection. Am J Hum Genet. 2021;108:1409–22.
Neveling K, Mantere T, Vermeulen S, Oorsprong M, van Beek R, Kater-Baats E, et al. Next-generation cytogenetics: Comprehensive assessment of 52 hematological malignancy genomes by optical genome mapping. Am J Hum Genet. 2021;108:1423–35.
Sahajpal NS, Barseghyan H, Kolhe R, Hastie A, Chaubey A. Optical genome mapping as a next-generation cytogenomic tool for detection of structural and copy number variations for prenatal genomic analyses. Genes. 2021;12:398.
Rack K, De Bie J, Ameye G, Gielen O, Demeyer S, Cools J, et al. Optimizing the diagnostic workflow for acute lymphoblastic leukemia by optical genome mapping. Am J Hematol. 2022;97:548–61.
Kanagal-Shamanna R, Singh RR, Routbort MJ, Patel KP, Medeiros LJ, Luthra R. Principles of analytical validation of next-generation sequencing based mutational analysis for hematologic neoplasms in a CLIA-certified laboratory. Expert Rev Mol Diagn. 2016;16:461–72.
Kanagal-Shamanna R, Orazi A, Hasserjian RP, Arber DA, Reichard K, Hsi ED, et al. Myelodysplastic/myeloproliferative neoplasms-unclassifiable with isolated isochromosome 17q represents a distinct clinico-biologic subset: a multi-institutional collaborative study from the Bone Marrow Pathology Group. Mod Pathol. 2021;35:470–9.
Kanagal-Shamanna R, Montalban-Bravo G, Sasaki K, Darbaniyan F, Jabbour E, Bueso-Ramos C, et al. Only SF3B1 mutation involving K700E independently predicts overall survival in myelodysplastic syndromes. Cancer. 2021;127:3552–65.
Kanagal-Shamanna R, Montalban-Bravo G, Katsonis P, Sasaki K, Class CA, Jabbour E, et al. Evolutionary action score identifies a subset of TP53 mutated myelodysplastic syndrome with favorable prognosis. Blood. Cancer J. 2021;11:52.
Mallo M, Sole F. Beyond morphology: to be or not to be an MDS. Br J Haematol. 2021;194:238–9.
Killick SB, Wiseman DH, Quek L, Cargo C, Culligan D, Enright H, et al. British Society for Haematology guidelines for the diagnosis and evaluation of prognosis of Adult Myelodysplastic Syndromes. Br J Haematol. 2021;194:282–93.
Kanagal-Shamanna R, Bueso-Ramos CE, Barkoh B, Lu G, Wang S, Garcia-Manero G, et al. Myeloid neoplasms with isolated isochromosome 17q represent a clinicopathologic entity associated with myelodysplastic/myeloproliferative features, a high risk of leukemic transformation, and wild-type TP53. Cancer. 2012;118:2879–88.
McGowan-Jordan J ISCN 2016: An International System for Human Cytogenomic Nomenclature (2016); Recommendations of the International Standing Human Committee on Human Cytogenomic Nomenclature Including New Sequence-based Cytogenomic. Karger, 2016.
Kanagal-Shamanna R, Yin CC, Miranda RN, Bueso-Ramos CE, Wang XI, Muddasani R, et al. Therapy-related myeloid neoplasms with isolated del(20q): comparison with cases of de novo myelodysplastic syndrome with del(20q). Cancer Genet. 2013;206:42–46.
Tang Z, Kanagal-Shamanna R, Tang G, Patel K, Medeiros LJ, Toruner GA. Analytical and clinical performance of chromosomal microarrays compared with FISH panel and conventional karyotyping in patients with chronic lymphocytic leukemia. Leuk Res. 2021;108:106616.
Genomics B. Bionano solve theory of operation: structural variant calling. Bionano Genomics. 2020.
Sahajpal NS, Mondal AK, Tvrdik T, Hauenstein J, Shi H, Deeb KK, et al. Optical genome mapping: clinical validation and diagnostic utility for enhanced cytogenomic analysis of hematological neoplasms. medRxiv 2022;2022.2003.2014.22272363.
Duncavage EJ, Schroeder MC, O’Laughlin M, Wilson R, MacMillan S, Bohannon A, et al. Genome sequencing as an alternative to cytogenetic analysis in myeloid cancers. N. Engl J Med. 2021;384:924–35.
Stengel A, Shahswar R, Haferlach T, Walter W, Hutter S, Meggendorfer M, et al. Whole transcriptome sequencing detects a large number of novel fusion transcripts in patients with AML and MDS. Blood Adv. 2020;4:5393–401.
Cluzeau T, Sebert M, Rahme R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the groupe francophone des myelodysplasies (GFM). J Clin Oncol. 2021;39:1575–83.
Cluzeau T, Loschi M, Fenaux P, Komrokji R, Sallman DA. Personalized medicine for TP53 mutated myelodysplastic syndromes and acute myeloid leukemia. Int J Mol Sci. 2021;22:10105.
Choi SM, Dewar R, Burke PW, Shao L. Partial tandem duplication of KMT2A (MLL) may predict a subset of myelodysplastic syndrome with unique characteristics and poor outcome. Haematologica. 2018;103:e131–4.
Sun QY, Ding LW, Tan KT, Chien W, Mayakonda A, Lin DC, et al. Ordering of mutations in acute myeloid leukemia with partial tandem duplication of MLL (MLL-PTD). Leukemia. 2017;31:1–10.
Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Arango Ossa JE, Nannya Y, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022;0:EVIDoa2200008.
Haferlach T, Hutter S, Meggendorfer M. Genome sequencing in myeloid cancers. N. Engl J Med. 2021;384:e106.
Dolnik A, Schrezenmeier J, Bullinger L. Genome sequencing in myeloid cancers. N. Engl J Med. 2021;384:e106.
Mack E, Kremer J, Neubauer A. Genome sequencing in myeloid cancers. N. Engl J Med. 2021;384:e106.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Pastor S, Tran O, Jin A, Carrado D, Silva BA, Uppuluri L, et al. Optical mapping of the 22q11.2DS region reveals complex repeat structures and preferred locations for non-allelic homologous recombination (NAHR). Sci Rep. 2020;10:12235.
Sabatella M, Mantere T, Waanders E, Neveling K, Mensenkamp AR, van Dijk F, et al. Optical genome mapping identifies a germline retrotransposon insertion in SMARCB1 in two siblings with atypical teratoid rhabdoid tumors. J Pathol. 2021;255:202–11.
Eisfeldt J, Pettersson M, Petri A, Nilsson D, Feuk L, Lindstrand A. Hybrid sequencing resolves two germline ultra-complex chromosomal rearrangements consisting of 137 breakpoint junctions in a single carrier. Hum Genet. 2021;140:775–90.
Acknowledgements
This work was supported in part by the Institutional Research Grant and leukemia SPORE career enhancement award (to RK-S). The reagents for the study were, in part, provided by Bionano Genomics.
Author information
Authors and Affiliations
Contributions
HY, GG-M, and RK-S designed the study. HY and RK-S collected study samples and patient data, performed the experiments, analyzed, and interpreted the data. KS performed the statistical analysis. HY, GG-M, KS, GM-B, ZT, YW, TK, KC, DR, HN, AK, MM, CB-R, HK, LJM, RL, and RK-S provided patient data, analyzed, and interpreted the results, provided scientific input and edited the manuscript. RK-S wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, H., Garcia-Manero, G., Sasaki, K. et al. High-resolution structural variant profiling of myelodysplastic syndromes by optical genome mapping uncovers cryptic aberrations of prognostic and therapeutic significance. Leukemia 36, 2306–2316 (2022). https://doi.org/10.1038/s41375-022-01652-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01652-8
This article is cited by
-
Scrambling the genome in cancer: causes and consequences of complex chromosome rearrangements
Nature Reviews Genetics (2024)
-
Optical Genome Mapping in MDS and AML as tool for structural variant profiling—comment and data update on Yang et al.: “High-resolution structural variant profiling of myelodysplastic syndromes by optical genome mapping uncovers cryptic aberrations of prognostic and therapeutic significance”
Leukemia (2023)
-
Myelodysplastic syndromes
Nature Reviews Disease Primers (2022)