Abstract
Objectives
To determine parental perspectives in a trial with waived consent.
Study design
Anonymous survey of birth parents with term infants who were randomized using a waiver of consent, administered after infant discharge.
Results
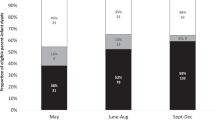
121 (11%) survey responses were collected. Of the 121 responding parents 111 (92%) reported that this form of consent was acceptable and 116 (96%) reported feeling comfortable having another child participate in a similar study. 110 (91%) respondents reported that they both understood the information provided in the consent process and had enough time to consider participation. Four percent had a negative opinion on the study’s effect on their child’s health.
Conclusions
Most responding parents reported both acceptability of this study design in the neonatal period and that the study had a positive effect on their child’s health. Future work should investigate additional ways to involve parents and elicit feedback on varied methods of pediatric consent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Katheria A, Schmölzer GM, Janvier A, Kapadia V, Saugstad OD, Vento M, et al. A narrative review of the rationale for conducting neonatal emergency studies with a waived or deferred consent approach. Neonatology. 2023;120:344–52.
Rich WD, Auten KJ, Gantz MG, Hale EC, Hensman AM, Newman NS, et al. Antenatal consent in the SUPPORT trial: challenges, costs, and representative enrollment. Pediatrics. 2010;126:e215–21.
Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA: J Am Med Assoc. 2019;322:1877–86.
Katheria AC, Allman P, Szychowski JM, Essers J, Carlo WA, Schmölzer GM, et al. Perinatal outcomes of subjects enrolled in a multicenter trial with a waiver of antenatal consent. Am J Perinatol. 2022;39:904–8.
Arnup SJ, McKenzie JE, Hemming K, Pilcher D, Forbes AB. Understanding the cluster randomised crossover design: a graphical illustration of the components of variation and a sample size tutorial. Trials. 2017;18:381.
Katheria AC, Clark E, Yoder B, Schmölzer GM, Yan Law BH, El-Naggar W, et al. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am J Obstet Gynecol. 2023;228:217.e211–7.e214.
Bjorland PA, Øymar K, Ersdal HL, Rettedal SI. Incidence of newborn resuscitative interventions at birth and short-term outcomes: a regional population-based study. BMJ Paediatr Open. 2019;3:e000592.
Schreiner MS, Feltman D, Wiswell T, Wootton S, Arnold C, Tyson J, et al. When is a waiver of consent appropriate in a neonatal clinical trial? Pediatrics. 2014;134:1006–12.
O’Shea N, Doran K, Ryan CA, Dempsey EM. Parental and clinician views of consent in neonatal research. Ir Med J. 2018;111:706.
Burgess E, Singhal N, Amin H, McMillan DD, Devrome H. Consent for clinical research in the neonatal intensive care unit: a retrospective survey and a prospective study. Arch Dis Child Fetal Neonatal Ed. 2003;88:F280–285.
Harron K, Woolfall K, Dwan K, Gamble C, Mok Q, Ramnarayan P, et al. Deferred consent for randomized controlled trials in emergency care settings. Pediatrics. 2015;136:e1316–22.
Ross CE, Lehmann S, Hayes MM, Yamin JB, Berg RA, Kleinman ME, et al. Community consultation in the pediatric intensive care unit for an exception from informed consent Trial: A survey of patient caregivers. Resusc. Plus. 2023;13:100355.
Rich WD, Katheria AC. Waiver of consent in a trial intervention occurring at birth—how do parents feel? Front Pediatr. 2017;5:56.
Weiss EM, Olszewski AE, Guttmann KF, Magnus BE, Li S, Shah AR, et al. Parental factors associated with the decision to participate in a neonatal clinical trial. JAMA Netw Open. 2021;4:e2032106.
Funding
Funding Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award Number R01HD096023. This trial is registered on ClinicalTrials.gov NCT# 03631940.
Author information
Authors and Affiliations
Contributions
AK and NF conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and revised the manuscript. AM, WR designed the data collection instruments, collected data, carried out the initial analyses, and critically reviewed and revised the manuscript. BM critically reviewed, revised the manuscript, and added an IRB perspective to the trial. GS; BL; BY; EC; WE, FV; and RD coordinated and supervised data collection at their individual sites, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
Dr. Katheria reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. No other disclosures were reported.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katheria, A.C., Schmölzer, G.M., Law, B. et al. Parental perspectives on a trial using waived informed consent at birth. J Perinatol 44, 415–418 (2024). https://doi.org/10.1038/s41372-023-01853-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01853-8