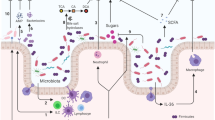
Abstract
Bacterial infections present a significant threat to neonates. Increasingly, studies demonstrate associations between human diseases and the microbiota, the communities of microorganisms on or in the body. A “healthy” microbiota with a great diversity and balance of microorganisms can resist harmful pathogens and protect against infections, whereas a microbiota suffering from dysbiosis, can predispose to pathogen colonization and subsequent infection. For decades, strategies such as bacterial interference, decolonization, prebiotics, and probiotics have been tested to reduce Staphylococcus aureus disease and other infections in neonates. More recently, microbiota transplant has emerged as a strategy to broadly correct dysbiosis, promote colonization resistance, and prevent infections. This paper discusses the benefits of a healthy neonate’s microbiota, exposures that alter the microbiota, associations of dysbiosis and neonatal disease, strategies to prevent dysbiosis, such as microbiota transplantation, and presents a framework of microbiome manipulation to reduce Staphylococcus aureus (S. aureus) and other infections in neonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5:31.
Zhao N, Khamash DF, Koh H, Voskertchian A, Egbert E, Mongodin EF, et al. Low diversity in nasal microbiome associated with Staphylococcus aureus colonization and bloodstream infections in hospitalized neonates. Open Forum Infect Dis. 2021;8:ofab475.
Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21:109–17.
Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–54.
Weyrich LS, Dixit S, Farrer AG, Cooper AJ, Cooper AJ. The skin microbiome: associations between altered microbial communities and disease. Aust J Dermatol. 2015;56:268–74.
Duar RM, Kyle D, Casaburi G. Colonization resistance in the infant gut: the role of B. infantis in reducing pH and preventing pathogen growth. High Throughput. 2020;9:7.
Hoang DM, Levy EI, Vandenplas Y. The impact of caesarean section on the infant gut microbiome. Acta Paediatr. 2021;110:60–7.
Parnanen KMM, Hultman J, Markkanen M, Satokari R, Rautava S, Lamendella R, et al. Early-life formula feeding is associated with infant gut microbiota alterations and an increased antibiotic resistance load. Am J Clin Nutr. 2022;115:407–21.
Reyman M, van Houten MA, Watson RL, Chu M, Arp K, de Waal WJ, et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nat Commun. 2022;13:893.
Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3:e00251–12.
Stressmann FA, Rogers GB, van der Gast CJ, Marsh P, Vermeer LS, Carroll MP, et al. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax. 2012;67:867–73.
Lee CC, Feng Y, Yeh YM, Lien R, Chen CL, Zhou YL, et al. Gut dysbiosis, bacterial colonization and translocation, and neonatal sepsis in very-low-birth-weight preterm infants. Front Microbiol. 2021;12:746111.
Graspeuntner S, Waschina S, Kunzel S, Twisselmann N, Rausch TK, Cloppenborg-Schmidt K, et al. Gut dysbiosis with Bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin Infect Dis. 2019;69:268–77.
Mukhopadhyay S, Lee JJ, Hartman E, Woodford E, Dhudasia MB, Mattei LM, et al. Preterm infants at low risk for early-onset sepsis differ in early fecal microbiome assembly. Gut Microbes. 2022;14:2154091.
Nurjadi D, Eichel VM, Tabatabai P, Klein S, Last K, Mutters NT, et al. Surveillance for colonization, transmission, and infection with methicillin-susceptible Staphylococcus aureus in a neonatal intensive care unit. JAMA Netw Open. 2021;4:e2124938.
Schwartz DJ, Shalon N, Wardenburg K, DeVeaux A, Wallace MA, Hall-Moore C, et al. Gut pathogen colonization precedes bloodstream infection in the neonatal intensive care unit. Sci Transl Med. 2023;15:eadg5562.
Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–91.
Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8.
Chi C, Buys N, Li C, Sun J, Yin C. Effects of prebiotics on sepsis, necrotizing enterocolitis, mortality, feeding intolerance, time to full enteral feeding, length of hospital stay, and stool frequency in preterm infants: a meta-analysis. Eur J Clin Nutr. 2019;73:657–70.
Sharif S, Oddie SJ, Heath PT, McGuire W. Prebiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2023;6:CD015133.
Patel RM, Underwood MA. Probiotics and necrotizing enterocolitis. Semin Pediatr Surg. 2018;27:39–46.
Chi C, Li C, Buys N, Wang W, Yin C, Sun J. Effects of probiotics in preterm infants: a network meta-analysis. Pediatrics. 2021;147:e20200706.
Zhang GQ, Hu HJ, Liu CY, Shakya S, Li ZY. Probiotics for preventing late-onset sepsis in preterm neonates: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine. 2016;95:e2581.
Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, McMaster Probiotic P, et al. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. 2020;159:467–80.
Bertelli C, Pillonel T, Torregrossa A, Prod’hom G, Fischer CJ, Greub G, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin Infect Dis. 2015;60:924–7.
Cavicchiolo ME, Magnani M, Calgaro S, Bonadies L, Castagliulo I, Morelli L, et al. Neonatal sepsis associated with Lactobacillus supplementation. J Perinat Med. 2019;48:87–88.
Chiang MC, Chen CL, Feng Y, Chen CC, Lien R, Chiu CH. Lactobacillus rhamnosus sepsis associated with probiotic therapy in an extremely preterm infant: pathogenesis and a review for clinicians. J Microbiol Immunol Infect. 2021;54:575–80.
Poindexter B, Committee on Fetus and Newborn. Use of probiotics in preterm infants. Pediatrics. 2021;147:e2021051485.
Govindarajan V, Devadas S, Shah PA, Diggikar S. Impact of kangaroo mother care on skin microbiome of very preterm infants - a pilot study. Indian J Pediatr. 2023. https://doi.org/10.1007/s12098-023-04562-4. Epub ahead of print.
Hendricks-Munoz KD, Xu J, Parikh HI, Xu P, Fettweis JM, Kim Y, et al. Skin-to-skin care and the development of the preterm infant oral microbiome. Am J Perinatol. 2015;32:1205–16.
Chan GJ, Valsangkar B, Kajeepeta S, Boundy EO, Wall S. What is kangaroo mother care? Systematic review of the literature. J Glob Health. 2016;6:010701.
Arya S, Chhabra S, Singhal R, Kumari A, Wadhwa N, Anand P, et al. Effect on neonatal sepsis following immediate kangaroo mother care in a newborn intensive care unit: a post-hoc analysis of a multicentre, open-label, randomised controlled trial. EClinicalMedicine. 2023;60:102006.
Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of fecal-derived microbiota transfer using orally administered capsules for recurrent Clostridium difficile infection. BMC Infect Dis. 2015;15:191.
Khan MY, Dirweesh A, Khurshid T, Siddiqui WJ. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:1309–17.
Yoon YK, Suh JW, Kang EJ, Kim JY. Efficacy and safety of fecal microbiota transplantation for decolonization of intestinal multidrug-resistant microorganism carriage: beyond Clostridioides difficile infection. Ann Med. 2019;51:379–89.
Nicholson MR, Mitchell PD, Alexander E, Ballal S, Bartlett M, Becker P, et al. Efficacy of fecal microbiota transplantation for clostridium difficile infection in children. Clin Gastroenterol Hepatol. 2020;18:612–9.e11.
Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, et al. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. 2020;183:324–34.e5.
Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22:250–3.
Committee Opinion No. 725: Vaginal Seeding. Obstet Gynecol. 2017;130:e274-e278.
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-7. Infect Control Hosp Epidemiol. 2020;41:1–18.
Ericson JE, Popoola VO, Smith PB, Benjamin DK, Fowler VG, Benjamin DK Jr, et al. Burden of invasive Staphylococcus aureus infections in hospitalized infants. JAMA Pediatr. 2015;169:1105–11.
Huang YC, Chou YH, Su LH, Lien RI, Lin TY. Methicillin-resistant Staphylococcus aureus colonization and its association with infection among infants hospitalized in neonatal intensive care units. Pediatrics. 2006;118:469–74.
Song X, Cheung S, Klontz K, Short B, Campos J, Singh N. A stepwise approach to control an outbreak and ongoing transmission of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. Am J Infect Control. 2010;38:607–11.
Gregory ML, Eichenwald EC, Puopolo KM. Seven-year experience with a surveillance program to reduce methicillin-resistant Staphylococcus aureus colonization in a neonatal intensive care unit. Pediatrics. 2009;123:e790–6.
Maraqa NF, Aigbivbalu L, Masnita-Iusan C, Wludyka P, Shareef Z, Bailey C, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization and infection among infants at a level III neonatal intensive care unit. Am J Infect Control. 2011;39:35–41.
Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129:e914–22.
Milstone AM, Voskertchian A, Koontz DW, Khamash DF, Ross T, Aucott SW, et al. Effect of treating parents colonized with Staphylococcus aureus on transmission to neonates in the intensive care unit: a randomized clinical trial. JAMA. 2020;323:319–28.
Khamash DF, Voskertchian A, Milstone AM. Manipulating the microbiome: evolution of a strategy to prevent S. aureus disease in children. J Perinatol. 2018;38:105–9.
Houck PW, Nelson JD, Kay JL. Fatal septicemia due to Staphylococcus aureus 502A. Report of a case and review of the infectious complications of bacterial interference programs. Am J Dis Child. 1972;123:45–8.
Uehara Y, Nakama H, Agematsu K, Uchida M, Kawakami Y, Abdul Fattah AS, et al. Bacterial interference among nasal inhabitants: eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J Hosp Infect. 2000;44:127–33.
Piewngam P, Khongthong S, Roekngam N, Theapparat Y, Sunpaweravong S, Faroongsarng D, et al. Probiotic for pathogen-specific Staphylococcus aureus decolonisation in Thailand: a phase 2, double-blind, randomised, placebo-controlled trial. Lancet Microbe. 2023;4:e75–83.
Heinrich N, Mueller A, Bartmann P, Simon A, Bierbaum G, Engelhart S. Successful management of an MRSA outbreak in a neonatal intensive care unit. Eur J Clin Microbiol Infect Dis. 2011;30:909–13.
Hitomi S, Kubota M, Mori N, Baba S, Yano H, Okuzumi K, et al. Control of a methicillin-resistant Staphylococcus aureus outbreak in a neonatal intensive care unit by unselective use of nasal mupirocin ointment. J Hosp Infect. 2000;46:123–9.
Jernigan JA, Titus MG, Groschel DH, Getchell-White S, Farr BM. Effectiveness of contact isolation during a hospital outbreak of methicillin-resistant Staphylococcus aureus. Am J Epidemiol. 1996;143:496–504.
Milstone AM, Budd A, Shepard JW, Ross T, Aucott S, Carroll KC, et al. Role of decolonization in a comprehensive strategy to reduce methicillin-resistant Staphylococcus aureus infections in the neonatal intensive care unit: an observational cohort study. Infect Control Hosp Epidemiol. 2010;31:558–60.
Milstone AM, Song X, Coffin S, Elward A. Identification and eradication of methicillin-resistant Staphylococcus aureus colonization in the neonatal intensive care unit: results of a national survey. Infect Control Hosp Epidemiol. 2010;31:766–8.
Delaney HM, Wang E, Melish M. Comprehensive strategy including prophylactic mupirocin to reduce Staphylococcus aureus colonization and infection in high-risk neonates. J Perinatol. 2013;33:313–8.
You JH, Chan CY, Wong MY, Ip M. Active surveillance and decolonization of methicillin-resistant Staphylococcus aureus on admission to neonatal intensive care units in Hong Kong: a cost-effectiveness analysis. Infect Control Hosp Epidemiol. 2012;33:1024–30.
Popoola VO, Colantuoni E, Suwantarat N, Pierce R, Carroll KC, Aucott SW, et al. Active surveillance cultures and decolonization to reduce Staphylococcus aureus infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2016;37:381–7.
Popoola VO, Milstone AM. Decolonization to prevent Staphylococcus aureus transmission and infections in the neonatal intensive care unit. J Perinatol. 2014;34:805–10.
Voskertchian A, Akinboyo IC, Colantuoni E, Johnson J, Milstone AM. Association of an active surveillance and decolonization program on incidence of clinical cultures growing Staphylococcus aureus in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2018;39:882–4.
Ristagno EH, Bryant KA, Boland LF, Stout GG, Junkins AD, Woods CR, et al. Effect of intranasal mupirocin prophylaxis on methicillin-resistant Staphylococcus aureus transmission and invasive staphylococcal infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2018;39:741–5.
Akinboyo IC, Voskertchian A, Gorfu G, Betz JF, Ross TL, Carroll KC, et al. Epidemiology and risk factors for recurrent Staphylococcus aureus colonization following active surveillance and decolonization in the NICU. Infect Control Hosp Epidemiol. 2018;39:1334–9.
Chapman AK, Aucott SW, Gilmore MM, Advani S, Clarke W, Milstone AM. Absorption and tolerability of aqueous chlorhexidine gluconate used for skin antisepsis prior to catheter insertion in preterm neonates. J Perinatol. 2013;33:768–71.
Chapman AK, Aucott SW, Milstone AM. Safety of chlorhexidine gluconate used for skin antisepsis in the preterm infant. J Perinatol. 2012;32:4–9.
Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect Control Hosp Epidemiol. 2010;31:846–9.
Acknowledgements
This work was supported in part by the National Institutes of Health under Award Numbers K24AI141580 (AMM), K23HD100594 (JJ), T32 AI052071 (ECP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
AA drafted the manuscript. All authors reviewed and provided substantial input to the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aneja, A., Johnson, J., Prochaska, E.C. et al. Microbiome dysbiosis: a modifiable state and target to prevent Staphylococcus aureus infections and other diseases in neonates. J Perinatol 44, 125–130 (2024). https://doi.org/10.1038/s41372-023-01810-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01810-5