Abstract
Background
Metabolic syndrome (MetS) is associated with premature aging, but whether this association is driven by genetic or lifestyle factors remains unclear.
Methods
Two independent discovery cohorts, consisting of twins and unrelated individuals, were examined (N = 268, aged 23–69 years). The findings were replicated in two cohorts from the same base population. One consisted of unrelated individuals (N = 1 564), and the other of twins (N = 293). Participants’ epigenetic age, estimated using blood DNA methylation data, was determined using the epigenetic clocks GrimAge and DunedinPACE. The individual-level linear regression models for investigating the associations of MetS and its components with epigenetic aging were followed by within-twin-pair analyses using fixed-effects regression models to account for genetic factors.
Results
In individual-level analyses, GrimAge age acceleration was higher among participants with MetS (N = 56) compared to participants without MetS (N = 212) (mean 2.078 [95% CI = 0.996,3.160] years vs. −0.549 [−1.053,−0.045] years, between-group p = 3.5E-5). Likewise, the DunedinPACE estimate was higher among the participants with MetS compared to the participants without MetS (1.032 [1.002,1.063] years/calendar year vs. 0.911 [0.896,0.927] years/calendar year, p = 4.8E-11). An adverse profile in terms of specific MetS components was associated with accelerated aging. However, adjustments for lifestyle attenuated these associations; nevertheless, for DunedinPACE, they remained statistically significant. The within-twin-pair analyses suggested that genetics explains these associations fully for GrimAge and partly for DunedinPACE. The replication analyses provided additional evidence that the association between MetS components and accelerated aging is independent of the lifestyle factors considered in this study, however, suggesting that genetics is a significant confounder in this association.
Conclusions
The results of this study suggests that MetS is associated with accelerated epigenetic aging, independent of physical activity, smoking or alcohol consumption, and that the association may be explained by genetics.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a significant precursor to cardiovascular diseases and type 2 diabetes [1]. MetS refers to the co-occurrence of several known cardiovascular risk factors that typically increase with age, such as insulin resistance, obesity, atherogenic dyslipidemia, and hypertension [2, 3]. The worldwide prevalence of adulthood MetS is approximately 30–40% [4, 5]. MetS is strongly linked to a lifestyle characterized by an unhealthy diet and physical inactivity [6], and it may lead to premature aging [7,8,9]. However, it is unclear whether the accumulation of MetS components increase the likelihood of developing diseases that shorten lifespan or if the accumulation of MetS components itself accelerates the aging process.
Epigenetics, particularly age-related changes in DNA methylation (DNAm), constitute the primary hallmark of biological aging [10, 11]. Epigenetic mechanisms regulate gene expression and help us adapt to different environments and lifestyles, including unhealthy diet and physical inactivity, which are associated with the increasing prevalence of MetS. Genome-wide DNAm data can be used to construct composite scores, i.e. epigenetic clocks, which provide an estimate of an individual’s biological age. Epigenetic clocks are algorithms that aim to quantify biological aging using DNAm levels at specific CpG sites. Epigenetic clocks summarize the effects of genetic susceptibility, as well as the cumulative effect of lifestyle and environmental factors, on physiological aging over the life course [12, 13].
The epigenetic clock GrimAge was developed to predict mortality [14]. Compared to previously developed clocks, GrimAge may best capture the DNAm changes associated with MetS and its components [9, 15]. The recently developed DunedinPACE estimator differs from GrimAge and other predecessors because it has been developed to predict the pace of aging measured over a 20-year follow-up. DunedinPACE operationalizes aging as a decline in physiological integrity over the years [16] and may, therefore, be a particularly good marker for assessing the effects of the age-related accumulation of MetS risk factors on epigenetic aging.
The epigenome is an intriguing target for both MetS and age-related physiological changes because it is a major determinant of gene expression that is modifiable by the environment and lifestyle. A more adverse metabolic risk profile, or MetS, is associated with accelerated epigenetic aging [8, 9, 15, 17,18,19]. However, the results vary by epigenetic clock, and to the best of our knowledge, no previous study has investigated the association of MetS with the most recent clock, DunedinPACE, and/or considered the effects of genetic factors. Genotype has an important effect on both the components of MetS and the epigenome [20], which means genetic confounding is possible when assessing the association between MetS and epigenetic aging. Thus, our objective was to investigate the cross-sectional association of MetS and its components with epigenetic aging. We employed two recent epigenetic clocks, GrimAge and DunedinPACE, in our analyses. To control for genotype and sex, age, and early childhood environmental factors shared by twin siblings, we employed within-twin-pair comparisons.
Methods
Study populations
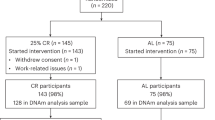
The data (N = 268, 57% female) for the primary, discovery-oriented analyses of this study were drawn from two Finnish population-based cohort studies: the Finnish Twin Cohort (FTC) [21,22,23,24] and the Estrogenic Regulation of Muscle Apoptosis (ERMA) study [25] (for details see Supplement 1). The age range of the pooled study population covered most adulthood, from 23 to 69 years. Those who fulfilled the criteria for having MetS constituted 21% of the participants.
Replication analyses
To validate our primary results, we replicated the analyses using two cohorts (Supplement 1). The individual-level analyses were replicated in a large, independent Finnish cohort study, The Young Finns Study (YFS) [26, 27], which consisted of 1 564 unrelated individuals (55% female) aged 34–49 years and of which 22% had MetS. The within-twin-pair analyses were replicated in a dataset of Essential Hypertension Epigenetics Study (EH-Epi) [23, 28], which consisted of 293 twins (61% female) aged 56–69 years and of which 32% had MetS.
Research ethics
Previously given consents covered our study (Supplement 2).
Epigenetic aging
Blood-based DNAm profiles were obtained using Illumina’s Infinium HumanMethylation450 BeadChip or the Infinium MethylationEPIC BeadChip (Illumina, San Diego, CA, USA). In our previous articles, we described the generation, preprocessing, and normalization of DNAm data [13, 29]. In this study’s analyses, we used the epigenetic clocks GrimAge [14] and DunedinPACE [16]. Recently, epigenetic clocks based on principal components (PCs) have been developed to bolster the reliability and validity of the clocks [30]. We produced PC-based GrimAge estimates using an R package (https://github.com/MorganLevineLab/PC-Clocks). Age acceleration in years (GrimAgeAA) was defined as the residual obtained from regressing the estimated epigenetic age on chronological age. In addition, we obtained PC-based GrimAge components (adjusted for age), including DNAm smoking pack–years, DNAm ADM, DNAm B2M, DNAm cystatin C, DNAm GDF15, DNAm leptin, DNAm PAI-1, and DNAm TIMP-1. DunedinPACE provided an estimate of the pace of aging in years per calendar year [16]. DunedinPACE was calculated using a publicly available R package (https://github.com/danbelsky/DunedinPACE).
Metabolic syndrome
MetS was determined according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) [31], which was updated by the American Heart Association and the National Heart Lung and Blood Institute in 2005 [32].
Components of metabolic syndrome
Waist circumference was measured at the midpoint between the lowest rib and the iliac crest by trained research nurses. Fasting high density lipoprotein (HDL) cholesterol, triglyceride, and fasting glucose levels were measured via blood samples taken from the participants after an overnight fast. Blood pressure was measured with a sphygmomanometer. Systolic and diastolic blood pressure were measured three or two times (for the FTC and ERMA, respectively), and the mean of these measurements was used in our analysis. More detailed information about the measurement methods used has been presented previously for the FTC [21,22,23] and ERMA [25, 33]. The use of cholesterol- and glucose-lowering medications as well as of antihypertensives was self-reported with brand names and confirmed by a physician or a nurse during a medical examination.
Other covariates
Alcohol consumption was calculated as the number of alcoholic drinks (1 drink = 12 g ethanol) consumed per week. Smoking was classified according to the following three categories: never, former, and current smoker. Current smokers included both daily and occasional smokers.
Physical activity. In the FTC, the Baecke questionnaire was used to assess physical activity [34]. Following three indexes; the work index, the sport index, and the leisure-time index, were calculated using 16 items. All responses were given on a five-point scale except for questions regarding the main occupation and the types of two main sports. In the original publication, the test–retest reliability scores of the work, sport, and leisure-time indices were 0.88, 0.81, and 0.74, respectively [34]. The Baecke questionnaire has been validated for cardiorespiratory fitness among Finnish twins [35]. For the analysis, the participants were divided into the following three groups of physical activity according to the sport index: low (Groups 1–2), medium (Group 3), and high physical activity (Groups 4–5).
For the ERMA study, the participants’ self-reported physical activity was measured using a single-question scale that included seven physical-activity-level categories ranging from necessary daily activities and routines to participation in competitive sports [36]. For the analysis, the participants were further divided into the groups of low (Groups 1–2), medium (Groups 3–4), and high physical activity (Groups 5–7). The test–retest reliability, concurrent validity against accelerometer-measured physical activity, and associations with several physical performance measurements have been reported previously [37].
Statistical analysis
We analyzed differences in epigenetic aging (age acceleration/pace of aging) between the participants with and without MetS using linear regression analyses adjusted for the within-pair dependency of twins (family relatedness), age, and sex (including the interaction term age*sex) (Release 16; Stata Corporation, College Station, TX, USA). In addition, we employed a linear regression analysis to assess the association between specific MetS components and epigenetic aging. The dependent variable was age acceleration/pace of aging, while the independent variable was one of the MetS components (waist circumference, HDL cholesterol, triglycerides, fasting glucose, systolic blood pressure, and diastolic blood pressure). For triglycerides and fasting glucose, a natural log transformation was performed due to the skewed distribution of the variables. Model 1 included an adjustment for the family relatedness, age, and sex (including an interaction term age*sex). Then, we carried out the analyses with multivariable adjustments. We adjusted Model 1 for smoking status, alcohol consumption, and physical activity level (Model 2). Finally, we adjusted Model 2 for medications (cholesterol, blood pressure, and blood glucose; Model 2 + medications). After the individual-level analyses, fixed-effects within-twin-pair regression models were conducted for all twin pairs, as well as separately for the monozygotic (MZ) and dizygotic (DZ) pairs. If an association between MetS components and accelerated epigenetic aging is observed in the co-twin control design, particularly in the MZ pairs, this provides strong evidence for an association between MetS and epigenetic aging, independent of the genetic and other shared effects. We present exact two-sided p values and set the nominal level of significance at p ≤ .05.
Results
Participant characteristics
The mean age of the participants was 40.0 years (SD 14.6). The correlation between chronological age and DNAm GrimAge (mean 53.1, SD 12.5) was 0.95, while the correlation with DunedinPACE was 0.40. Furthermore, age acceleration (GrimAgeAA) exhibited a correlation of 0.61 with the pace of aging (DunedinPACE). The characteristics of the study participants, stratified by MetS status, are presented in Table 1. In total, 56 participants (21%) met the criteria for having MetS, with 59% being women. The mean age of the participants with MetS was 52.6 years (SD 15.6), ranging from 23 to 69 years.
Differences in epigenetic aging according to MetS status: individual-level analyses
Figure 1 presents the differences in age acceleration/pace of aging by MetS status for all participants. GrimAgeAA was higher among participants with MetS (n = 56) compared to participants without MetS (n = 212) (mean 2.078 [95% CI = 0.996, 3.160] years vs. −0.549 [−1.053, −0.045] years, between-group p = 3.5E-5). Likewise, the DunedinPACE estimate was higher among the participants with MetS compared to the participants without MetS (1.032 [1.002, 1.063] years/calendar year vs. 0.911 [0.896, 0.927] years/calendar year, p = 4.8E-11) (Supplementary Table 1).
Association between MetS components and epigenetic aging: individual-level analyses
An adverse profile of MetS components was associated with accelerated epigenetic aging. In Model 1 (adjusted for age and sex), all MetS components except for blood pressure were associated with GrimAgeAA. More specifically, greater waist circumference (standardized regression coefficient β = 0.235, p = 2.6E-4), higher levels of triglycerides (0.218, p = 2.6E-4) and fasting glucose (0.163, p = .027), and a lower level of HDL cholesterol (−0.231, p = .001) were associated with higher GrimAgeAA. Further adjustments for lifestyle factors (Model 2) and medication (Model 2 + medication) attenuated these associations to nonsignificant levels (Table 2).
In Model 1, all MetS components except for systolic blood pressure were associated with the DunedinPACE estimate. Greater waist circumference (0.349, p = 1.0E-7), higher levels of triglycerides (0.255, p = 3.1E-5), fasting glucose (0.264, p = 2.5E-4), and diastolic blood pressure (0.171, p = .017), and a lower level of HDL cholesterol (−0.296, p = 1.3E-6) were associated with higher DunedinPACE estimates. Further adjustments for lifestyle factors (Model 2) and medication (Model 2 + medication) attenuated these associations, which, however, remained statistically significant (Table 3).
Association between MetS components and epigenetic aging: within-twin-pair analyses
The results of the fixed-effects within-twin-pair regression analyses are presented in Table 4 for GrimAge and in Table 5 for DunedinPACE. For all twin pairs (Table 4a), the specific MetS components were not associated with GrimAgeAA. However, an adverse profile in terms of MetS components (except for fasting glucose and systolic blood pressure) was associated with an accelerated pace of aging (DunedinPACE) (Table 5a). More specifically, in the base model (naturally adjusted for age and sex), greater waist circumference (unstandardized regression coefficient β = 0.002, p = .004), higher levels of triglycerides (0.048, p = .007), diastolic blood pressure (0.003, p = .005), and a lower level of HDL cholesterol (−0.064, p = .007) were associated with a higher DunedinPACE estimate. After further adjustments for lifestyle factors and medication, these associations were attenuated to nonsignificant levels (except for triglycerides).
The fixed-effects within-twin-pair regression analyses were conducted separately for the MZ and DZ twin pairs. Greater waist circumference (0.001, p = .046) and higher levels of triglycerides (0.045, p = .037) were associated with higher DunedinPACE estimates among the MZ twin pairs (Table 5b). Lower levels of HDL cholesterol were associated with higher DunedinPACE estimates among the DZ twin pairs (Table 5c). After further adjustments for lifestyle factors and medication, these associations were attenuated to nonsignificant levels.
Replication analysis
The results of the replication analysis are presented as supplementary material (Supplement 3 and 4). The individual-level results derived from the YFS data (Supplement 3) were apparently similar to those derived from the two discovery cohorts, providing additional evidence that the association between MetS components and accelerated aging is independent of lifestyle factors considered in this study. The within-twin-pair results derived from the EH-Epi data (Supplement 4) suggest that genetics fully explain these associations not only for GrimAge but also for DunedinPACE. When using DunedinPACE, these results consistently showed weaker associations between MetS components and accelerated aging among MZ pairs, who share all their genetic variation, compared to DZ pairs, who share only 50%. This suggests that genetics is a significant confounding factor in this association.
Discussion
This study investigated the association between MetS and epigenetic aging using two epigenetic clocks, GrimAge and DunedinPACE, in a study population that covered the adult lifespan. We employed a co-twin control study design, which is a powerful setting for controlling for genetic and familial confounding. The analyses were replicated in two cohorts from the same base population. To the best of our knowledge, this is the first study to report the association between MetS and novel epigenetic clock DunedinPACE, and/or considering the effects of genetic factors. Our pioneering findings suggest that MetS is associated with an accelerated pace of aging, as measured with DunedinPACE. This study demonstrates for the first time that the link between MetS and premature aging may be explained by genetics.
Our individual-level analyses revealed that epigenetic aging was accelerated among participants with MetS compared to those without MetS, irrespective of age and sex, which indicates that biological aging accelerates even before the onset of MetS-related chronic diseases. More precisely, epigenetic aging was accelerated by 2.6 years (GrimAge) and 0.12 years/calendar year (DunedinPACE) among participants with MetS compared to those without MetS. In addition, we found that an adverse profile in terms of individual MetS components was associated with accelerated aging, with waist circumference exhibiting the strongest association. Our results suggest that the association between accelerated aging and blood pressure is weaker compared to other MetS components. This may be explained by the relatively high number (11.2%) of participants taking antihypertensive medications. The results derived from the replication of the individual-level analyses in a large Finnish cohort study were apparently similar to those derived from the primary analyses, providing additional evidence that also high blood pressure is associated with accelerated aging. These findings are in line with previous research related to the association between MetS and epigenetic aging [8, 9, 15, 17,18,19, 38].
Based on our preliminary analyses using older generation clocks (data not shown) and prior literature, we opted to utilize epigenetic clocks, GrimAge and DunedinPACE, in our research. Previous studies using both older generation clocks and the GrimAge clock have suggested that GrimAge may best capture the DNAm changes associated with MetS and its components [9, 15]. It is noteworthy that the GrimAge clock is estimated based on seven DNAm surrogate markers, including leptin, which is associated with obesity [39], and may thus be more suitable than older generation clocks for estimating the association between age acceleration and metabolic features. However, in this study, we found stronger associations using DunedinPACE, which was trained to predict the pace of aging using longitudinal data based on physiological aging measures. Therefore, DunedinPACE can be a particularly good marker for assessing the effects of the age-related accumulation of risk factors for MetS on epigenetic aging.
The exact mechanisms through which MetS may accelerate aging remain unclear, but they are likely related to physiological responses to excess fat accumulation [6, 40]. Obesity is considered pro-aging because it is associated with increased oxidative stress and a proinflammatory state, which, in turn, enhance white blood cell turnover [41]. It has been suggested that excess reactive oxygen species may contribute to metabolic dysregulation, cell damage, and consequently aging [42]. Meanwhile, HDL cholesterol may modulate epigenetic aging processes due to its antiatherogenic effects, such as the removal of lipid deposits, which are accompanied by a reduction in cytotoxic effects [43]. Furthermore, HDL reduces oxidative stress in plasma and cellular compartments, and the signaling pathways in which it participates are interconnected with stress response and survival pathways [43]. The effects of oxidative stress on the metabolic dysregulation seen in MetS may be partially mediated by DNAm [44]. Although our study did not demonstrate a clear association between high blood pressure and epigenetic aging, it is well known that high blood pressure has numerous unfavorable effects on biological aging [45]. Several key mechanisms, such as inflammation and oxidative stress, are common to both biological aging and the development of high blood pressure.
In this study, we investigated the association between MetS components and different DNAm-based surrogate biomarkers for health-related plasma proteins to gain more precise information about the underlying mechanisms explaining the associations (see Supplementary Table 2). DNAm pack-years and DNAm plasminogen activator inhibitor, PAI-1, exhibited the strongest associations with MetS components. Smoking behavior is a significantly stronger predictor of DNAm age than other lifestyle factors, particularly when using the GrimAge algorithm for estimation [28, 46]. Furthermore, it is well documented that smoking is associated with metabolic abnormalities and increases the risk of MetS [47]. Our findings are in line with previous research [9, 19] supporting the role of DNAm PAI-1 as a major driver in the association of the GrimAge clock with MetS and its features. This is reasonable, as MetS-related increases in cytokines and free fatty acids increase the production of PAI-1 by the liver, which complements the overproduction of PAI-1 by adipose tissue [6].
Previous literature suggests that the rising prevalence of MetS can be explained by the obesogenic environment; therefore, it is urgent that researchers identify the epigenetic mechanisms mediating the environmental impact on MetS etiology to recommend appropriate therapies and intervention strategies [20]. In our study, in addition to age and sex, we were able to acknowledge the effects of smoking, alcohol consumption, and physical activity level, which are known to affect both DNAm and MetS etiology [6, 13, 47,48,49]. Interestingly, in the primary individual-level analyses of the study, these lifestyle factors explained the associations of MetS components with the GrimAge clock but not with DunedinPACE. However, in the replication of the individual-level analyses, the associations, which were stronger for DunedinPACE compared to GrimAge, were significant for both clocks independent of the influence of lifestyle factors. This provides additional evidence that the association between MetS components and accelerated aging is independent of the lifestyle factors considered in this study.
A major strength of the present study was its co-twin control design, which naturally controls for age, sex, year of birth, and familial factors (both genetic and nongenetic) that are shared within twin pairs and may affect both exposure and outcome. To the best of our knowledge, no previous study has acknowledged the effect of genetics in estimating the associations between epigenetic aging and MetS, even though genotype has an important influence on both MetS components and the epigenome [20]. Our approach allows to control for genetic confounding when assessing the association between MetS and epigenetic aging. The results derived from the primary within-twin-pair analyses suggested that of the MetS components, waist circumference and triglycerides are associated with the pace of aging irrespective of genetics. In contrast, the results indicated that the association between MetS and epigenetic aging measured using the GrimAge algorithm might be more influenced by genetic confounding. The within-twin-pair replication analyses indicated that genetics fully explain these associations for both GrimAge and DunedinPACE, providing additional evidence that genetics is a major confounder in the association between MetS and epigenetic aging.
In addition, one strength of our study was that the study population covered the age range from young adulthood to older individuals. In the primary analysis, we investigated the association between MetS and epigenetic aging among a study population aged 23–69 years. The results of the replication analyses, which included middle-aged (YFS study) or older (EH-Epi) participants representing the general population with a narrow age range, were similar to those of the primary analysis.
Because of the cross-sectional study design, we could not draw any causal conclusions. The findings of this study concern the Finnish population, which is representative of high-income populations of European ancestry. We cannot draw any firm conclusions on how our findings apply to different ethnic groups and socioeconomic circumstances. The lifestyle factors acknowledged in the study did not include, for example, the effects of diet or work-related stress factors, such as shift work, on the association between MetS and accelerated epigenetic aging. Given the complex and partially unclear pathogenesis of MetS and its components, it is reasonable to use blood-based clocks, which assess systemic age acceleration, in investigating the association between MetS and epigenetic age acceleration. However, it should be noted that we cannot draw conclusions regarding whether MetS is associated with tissue- or cell-specific age acceleration.
In conclusion, this study demonstrates for the first time that genetic factors play a significant role in influencing the relationship between MetS components and epigenetic aging. More research is needed to determine which lifestyle factors may potentially mediate or moderate the association between MetS and epigenetic aging. Understanding the effects of different MetS components on epigenetic aging may lead to interventions that can slow down the aging process and prevent age-related diseases.
Availability of data and materials
All twin data used in this study can be found within the Biobank of the National Institute for Health and Welfare, Finland. All biobanked data are publicly available for use by qualified researchers following a standardized application procedure.
Pseudonymized ERMA datasets are available on reasonable request. To request the data, please contact Dr. Eija Laakkonen (eija.k.laakkonen@jyu.fi).
The YFS dataset comprises health-related participant data, which means that their use is restricted under the regulations on professional secrecy (Act on the Openness of Government Activities, 612/1999) and on sensitive personal data (Personal Data Act, 523/1999, implementing the EU data protection directive 95/46/EC). Due to these legal restrictions, the YFS data cannot be stored in public repositories or otherwise made publicly available. However, data access may be permitted on a case-by-case basis upon request. Data sharing outside the group is done in collaboration with the YFS group and requires a data-sharing agreement. Investigators can submit an expression of interest to the chairman of the publication committee (Prof. Mika Kähönen, Tampere University, Finland, or Prof. Terho Lehtimäki in relation to epigenetic and genetic data).
References
Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72.
Fadini GP, Ceolotto G, Pagnin E, de Kreutzenberg S, Avogaro A. At the crossroads of longevity and metabolism: the metabolic syndrome and lifespan determinant pathways. Aging Cell. 2011;10:10–17.
Hui WS, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–84.
Haverinen E, Paalanen L, Palmieri L, Padron-Monedero A, Noguer-Zambrano I, Sarmiento Suárez R, et al. Comparison of metabolic syndrome prevalence using four different definitions – a population-based study in Finland. Arch Public Health. 2021;79:231.
Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323:2526–28.
Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365:1415–28.
Révész D, Milaneschi Y, Verhoeven JE, Lin J, Penninx BWJH. Longitudinal associations between metabolic syndrome components and telomere shortening. J Clin Endocrinol Metab. 2015;100:3050–9.
Nannini DR, Joyce BT, Zheng Y, Gao T, Liu L, Yoon G, et al. Epigenetic age acceleration and metabolic syndrome in the coronary artery risk development in young adults study. Clin Epigenetic. 2019;11:160.
Lee HS, Park T. The influences of DNA methylation and epigenetic clocks, on metabolic disease, in middle-aged Koreans. Clin Epigenetics. 2020;12:148.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Schmauck-Medina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Borland Madsen H, et al. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging. 2022;14:6829–39.
Föhr T, Törmäkangas T, Lankila H, Viljanen A, Rantanen T, Ollikainen M, et al. The association between epigenetic clocks and physical functioning in older women: a 3-year follow-up. J Gerontol A Biol Sci Med Sci. 2022;77:1569–76.
Kankaanpää A, Tolvanen A, Bollepalli S, Leskinen T, Kujala UM, Kaprio J, et al. Leisure-time and occupational physical activity associates differently with epigenetic aging. Med Sci Sports Exerc. 2021;53:487–95.
Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11:303–27.
Arpón A, Milagro FI, Santos JL, García-Granero M, Riezu-Boj JI, Martínez JA. Interaction among sex, aging, and epigenetic processes concerning visceral fat, insulin resistance, and dyslipidaemia. Front Endocrinol. 2019;10:496.
Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife. 2022;11:e73420.
Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–46.
Irvin MR, Aslibekyan S, Do A, Zhi D, Hidalgo B, Claas SA, et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clin Epigenetics. 2018;10:56.
Sanchez D, Jeong A, Essé C, Bassa FK, Koné S, Acka F, et al. Validity and cardio-metabolic risk profiles of DNA methylation clocks among adults in south-central Côte d’Ivoire. Epigenetics Commun. 2022;2:1.
Carson C, Lawson HA. Epigenetics of metabolic syndrome. Physiol Genomics. 2018;50:947–55.
Kaidesoja M, Aaltonen S, Bogl LH, Heikkilä K, Kaartinen S, Kujala UM, et al. FinnTwin16: a longitudinal study from age 16 of a population-based finnish twin cohort. Twin Res Hum Genet. 2019;22:530–39.
Rose RJ, Salvatore JE, Aaltonen S, Barr PB, Bogl LH, Byers HA, et al. FinnTwin12 cohort: an updated review. Twin Res Hum Gen. 2019;22:302–11.
Kaprio J, Bollepalli S, Buchwald J, Iso-Markku P, Korhonen T, Kovanen V, et al. The older finnish twin cohort — 45 years of follow-up. Twin Res Hum Gen. 2019;22:240–54.
Naukkarinen J, Rissanen A, Kaprio J, Pietiläinen KH. Causes and consequences of obesity: the contribution of recent twin studies. Int J Obes. 2012;36:1017–24.
Kovanen V, Aukee P, Kokko K, Finni T, Tarkka IM, Tammelin T, et al. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women’s cohort: novel results show menopause-related differences in blood count. Menopause. 2018;25:1020–32.
Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, et al. Cohort profile: the cardiovascular risk in young finns study. Int J Epidemiol. 2008;37:1220–26.
Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85.
Drouard G, Ollikainen M, Mykkänen J, Raitakari O, Lehtimäki T, Kähönen M, et al. Multi-omics integration in a twin cohort and predictive modeling of blood pressure values. OMICS. 2022;26:130–41.
Kankaanpää A, Tolvanen A, Saikkonen P, Heikkinen A, Laakkonen EK, Kaprio J, et al. Do epigenetic clocks provide explanations for sex differences in life span? a cross-sectional twin study. J Gerontol A Biol Sci Med Sci. 2022;77:1898–906.
Higgins-Chen AT, Thrush KL, Wang Y, Minteer CJ, Kuo PL, Wang M, et al. A computational solution for bolstering reliability of epigenetic clocks: implications for clinical trials and longitudinal tracking. Nat Aging. 2022;2:644–61.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112:2735–52.
Hyvärinen M, Juppi HK, Taskinen S, Karppinen JE, Karvinen S, Tammelin TH, et al. Metabolic health, menopause, and physical activity—a 4-year follow-up study. Int J Obes. 2022;46:544–54.
Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42.
Mustelin L, Latvala A, Pietiläinen KH, Piirilä P, Sovijärvi AR, Kujala UM, et al. Associations between sports participation, cardiorespiratory fitness, and adiposity in young adult twins. J Appl Physiol. 2011;110:681–86.
Hirvensalo M, Lampinen P, Rantanen T. Physical exercise in old age: an eight-year follow-up study on involvement, motives, and obstacles among persons age 65–84. J Aging Phys Act. 1998;6:157–68.
Kekäläinen T, Laakkonen EK, Terracciano A, Savikangas T, Hyvärinen M, Tammelin TH, et al. Accelerometer-measured and self-reported physical activity in relation to extraversion and neuroticism: a cross-sectional analysis of two studies. BMC Geriatr. 2020;20:264.
Lundgren S, Kuitunen S, Pietiläinen KH, Hurme M, Kähönen M, Männistö S, et al. BMI is positively associated with accelerated epigenetic aging in twin pairs discordant for body mass index. J Intern Med. 2022;292:627–40.
Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients. 2019;11:2704.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50.
Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745.
Bonomini F, Rodella LF, Rezzani R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015;6:109–20.
Walter M. Interrelationships among hdl metabolism, aging, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1244–50.
Yara S, Lavoie JC, Levy E. Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics. 2015;7:283–300.
Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111.
Föhr T, Waller K, Viljanen A, Sanchez R, Ollikainen M, Rantanen T, et al. Does the epigenetic clock GrimAge predict mortality independent of genetic influences: an 18 year follow-up study in older female twin pairs. Clin Epigenetics. 2021;13:128.
Cena H, Fonte ML, Turconi G. Relationship between smoking and metabolic syndrome. Nutr Rev. 2011;69:745–53.
Sun K, Ren M, Liu D, Wang C, Yang C, Yan L. Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr. 2014;33:596–602.
Klopack ET, Carroll JE, Cole SW, Seeman TE, Crimmins EM. Lifetime exposure to smoking, epigenetic aging, and morbidity and mortality in older adults. Clin Epigenetics. 2022;14:72.
Acknowledgements
TF is supported by the Juho Vainio Foundation and the Yrjö Jahnsson Foundation. ES is supported by the Academy of Finland (grant numbers 341750, 346509, 260001), the Juho Vainio Foundation, the Päivikki and Sakari Sohlberg Foundation, and the Yrjö Jahnsson Foundation (6168). JK is supported by the Academy of Finland (grant numbers 213506, 308248, 312073, 336823), EC FP5 GenomEUtwin, NIH NIH/NHLBI (grant HL104125), EC MC ITN Project EPITRAIN, and the Sigrid Juselius Foundation. MO is supported by the Academy of Finland (grant numbers 328685, 307339, 297908 and 251316), EC MC ITN Project EPITRAIN, University of Helsinki Research Funds, and the Sigrid Juselius Foundation. KHP is supported by the Academy of Finland (grant numbers 272376, 266286, 314383, 335443), Finnish Medical Foundation, Finnish Diabetes Research Foundation, Novo Nordisk Foundation (NNF10OC1013354, NNF17OC0027232, NNF20OC0060547), Signe and Ane Gyllenberg Foundation, Sigrid Juselius Foundation, Government Research Funds for Helsinki University Hospital Research Funds, and the University of Helsinki. EKL was supported by the Academy of Finland (grant numbers 275323 and 330281) and the PACTS2 profiling action of the University of Jyväskylä funded by the Academy of Finland (326242). The Gerontology Research Center is a joint effort between the University of Jyväskylä and the University of Tampere. The YFS has been financially supported by the following sources: Academy of Finland (grant numbers 356405, 322098, 286284, 134309 (Eye), 126925, 121584, 124282, 255381, 256474, 283115, 319060, 320297, 314389, 338395, 330809, and 104821, 129378 (Salve), 117797 (Gendi), and 141071 (Skidi)); the Social Insurance Institution of Finland; Competitive State Research Financing of the Expert Responsibility Area of Kuopio, Tampere and Turku University Hospitals (grant X51001); the Juho Vainio Foundation; the Paavo Nurmi Foundation; the Finnish Foundation for Cardiovascular Research; the Finnish Cultural Foundation; the Sigrid Juselius Foundation; the Tampere Tuberculosis Foundation; the Emil Aaltonen Foundation; the Yrjö Jahnsson Foundation; the Signe and Ane Gyllenberg Foundation; the Diabetes Research Foundation of Finnish Diabetes Association; EU Horizon 2020 (grant 755320 for TAXINOMISIS and grant 848146 for To Aition); the European Research Council (grant 742927 for MULTIEPIGEN project); the Tampere University Hospital Supporting Foundation, Finnish Society of Clinical Chemistry; the Cancer Foundation Finland; pBETTER4U_EU (Preventing obesity through Biologically and bEhaviorally Tailored inTERventions for you; project number: 101080117); and the Jane and Aatos Erkko Foundation. The EH-Epi has been finally supported by the NIH/NHLBI grant HL104125.
Funding
Open Access funding provided by University of Jyväskylä (JYU).
Author information
Authors and Affiliations
Contributions
TF, ES, and MO designed the study. AK generated the epigenetic age estimates and contributed to data interpretation and result visualization. TF and AH conducted the analysis, interpreted the data, and drafted the first version of the manuscript. ES and JK supervised the statistical analyses and participated in data interpretation. ES contributed significantly to the writing process. UK, JK, KHP, and MO designed and collected the FTC dataset, and EKL built the ERMA dataset. MK, TL, and OR designed and collected the YFS dataset. XW designed and collected the EH-Epi dataset. All authors have been involved in the drafting and revision of the manuscript in terms of important intellectual content. They have approved the analysis performed and have given the final approval for the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Föhr, T., Hendrix, A., Kankaanpää, A. et al. Metabolic syndrome and epigenetic aging: a twin study. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01466-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01466-x