Abstract
Background:
Patients with heterotaxy syndrome, commonly associated with complex congenital heart disease (CHD), exhibit a higher risk of severe bacterial infection (SBI). We sought to define the change of a novel immunologic marker, the immunoglobulin M (IgM) memory B-cell percentage, and its association with SBI.
Methods:
We enrolled 46 (M/F 29/17) heterotaxy syndrome patients (42 right atrial isomerism (RAI) and 4 left atrial isomerism (LAI)) aged > 1 y during the period 2010–2012 in a tertiary care center. We analyzed IgM+CD27+ memory B-cell percentages. Patients with simple and complex CHD served as controls.
Results:
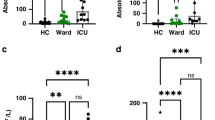
The mean IgM memory B-cell percentages were the lowest in the heterotaxy syndrome group, compared with those in complex and simple CHD groups (1.8 ± 2.1 vs. 3.9 ± 3.2 vs. 5.1 ± 4.7, P < 0.001). In the heterotaxy syndrome group, 41.3% had low IgM memory B-cell percentages (<1% of B cells). Seven had a history of community-acquired SBI and 85.7% of these had low IgM memory B-cell percentages, which was the only significant factors related to community-acquired SBI (P = 0.028).
Conclusion:
The memory B cell and IgM memory B-cell percentages are low in patients with heterotaxy syndrome, and the presence of IgM memory B-cell percentage < 1% correlates with community-acquired SBI.
Similar content being viewed by others
Main
Heterotaxy syndrome is a developmental defect involving abnormal arrangement of the left-right axis, which involves the thoracic and visceral organs. Because of associated abnormalities in cardiac looping, complex congenital heart disease (CHD) is common in heterotaxy patients, most of whom present with cyanosis and heart failure symptoms early in life and require multistage operations for long-term survival (1,2,3,4,5). Therefore, cardiac- and operation-related causes account for 60% of death in heterotaxy patients. Infection-related mortality is another major cause of death (6). Maldevelopment of the spleen is common in heterotaxy patients, and is an important cause of immunodeficiency in young children with heterotaxy (5,6,7,8). The incidence of heterotaxy syndrome is 1–1.24% of CHD patients (9,10), and can be further divided into right atrial isomerisms (RAI) and left atrial isomerisms (LAI) according to the atrial appendage morphology. In RAI patients, agenesis of the spleen is common, occurring in approximately 75% of these patients (4). Fulminant-encapsulated bacterial infection (particularly pneumococcus and Hemophilus influenza) is a common complication in asplenic patients (5,6,8). For LAI, approximately 43% of patients present with polysplenia and 10% of them present with asplenia (4). However, LAI patients also exhibit a higher rate of sepsis, which may be related to abnormal splenic function (8). In our previous large cohort study, we demonstrated that community-acquired severe bacterial infection (SBI) is common among heterotaxy patients, and that the incidence is significantly higher than that among patients with other cause of complex CHD (6). We also found a patient experiencing multiple SBI episodes is common, indicating that some heterotaxy patients have a higher risk of infection than those of others (6). Therefore, developing diagnostic methods for identifying such highly susceptible patients is crucial.
The risk of SBI in heterotaxy patients is highest before 2 y of age, and decreases gradually with age (6,11), possibly because lymphoid organs, such as the lymph nodes and enteric lymphoid tissue, may substitute for the splenic immune function in some patients as age increases (12). Although the spleen is a vital immunologic organ, there is still no simple and effective method for evaluating its function (13). Studies have demonstrated that the IgM+IgD+ memory B cells correspond closely to the circulating splenic marginal zone B cells (14). These IgM+IgD+ memory B cells forms the T independent pathway, which is responsible for the immune mechanisms that are activated in response to encapsulated bacteria. The percentage of IgM memory B cells measured using flow cytometry from the peripheral blood declines significantly after splenectomy, indicating that this marker is related to splenic immune function (15,16). Whether this marker can be used to predict susceptibility to SBI in heterotaxy patients remains unknown. Therefore, this study sought to elucidate the changes of this new marker and its correlation with SBI episodes.
Results
Basic Clinical Characteristics
Table 1 presents a summary of the basic clinical characteristics of 46 heterotaxy patients (42 with RAI and four with LAI), 63 complex CHD patients, and 66 simple CHD patients. Male predominance was found in the heterotaxy group, which was consistent with the findings of our previous study (6). The female predominance in the control group indicated that half of them were patients with an atrial septal defect. The age and oxygen saturation revealed no significant difference between the heterotaxy and complex CHD groups. The community-acquired SBI rate was 15.2% in the heterotaxy patients, which is significantly higher than that of the other two groups (7.9 and 0% in the complex and simple CHD groups, respectively; P = 0.007). The culture-positive SBI rate was also significantly higher in the heterotaxy group than in the CHD groups ( Table 1 ).
Memory B Cell and IgM Memory B-Cell Percentages
The CD 19+ B-lymphocyte percentages were similar among the three groups ( Table 1 ). In the B-lymphocyte subset, the heterotaxy patients had significantly fewer memory B cells (CD22+CD27+) than did the controls ( Figures 1a and 2a ). As shown in Table 1 , the mean percentage of memory B cells in the heterotaxy patients is 8.1 ± 7.2%, which is significantly lower than the mean percentages in the complex CHD (12.8 ± 10.1%) and simple CHD patients (15.2 ± 10.3%). Age, sex, and oxygen saturation were not associated with memory B-cell count in either the control or heterotaxy patients.
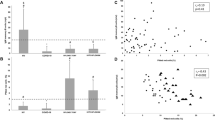
Flow cytometry analysis of IgM memory B cell in RAI (with or without previous SBI, complex CHD, and control group. Flow cytometry data of an (a) right atrial isomerism (RAI) patient with previous severe bacterial infection (SBI); (b) an RAI patient without previous SBI; (c) a patient with complex congenital heart disease (CHD); and (d) the simple CHD group (from left to right). Upper portion is the peripheral blood mononuclear cells stained with antibodies to CD19, CD22, and CD27 by using 3-color flow cytometry. Data are illustrated as a density plot. The memory B cells (CD22+27+) are denoted as blue rectangles, and were 1.1, 6.3, 21.5, and 16.4% of CD22+ cell for panel (a) to panel (d), respectively. Lower portion is the peripheral blood mononuclear cells stained with antibodies to CD22, CD27, IgM, and IgD, and analyzed using 4-color flow cytometry. The memory B cells (CD22+CD27+) were gated for further analysis. The memory B cells were divided into two groups based on IgM and IgD staining. The upper-right part, delineated by red rectangles, illustrates IgM memory B cells (IgM+IgD+), and the lower-left part (IgM-IgD-) indicates switched memory B cells. The IgM memory B cells as a percentage of CD22+ B cells are 0.09, 1.29, 10.2, and 5.4% from panel (a) to panel (d), respectively. Considerably lower memory B cell and IgM memory B-cell percentages are apparent in the RAI patients with previous SBI, in contrast to the RAI patients without previous SBI and other two groups.
Memory B and IgM memory B cell percentage in three groups of patients and in heterotaxy patients with or without previous SBI. (a) The memory B-cell percentage and (b) IgM memory B-cell percentage among the heterotaxy, complex and simple congenital heart disease groups. The memory B-cell percentage and IgM memory B-cell percentage are both significantly lower in the heterotaxy group, compared with those of the other two groups. (c) The memory B-cell percentage and (d) IgM memory B-cell percentage of patients with previously community-acquired severe bacterial infection (SBI), and those without community-acquired SBI, in heterotaxy patients. Both parameters are considerably lower and near zero in patients with previously community-acquired SBI.
The IgM memory B-cell (IgM+IgD+CD22+CD27+) percentage was lower in the heterotaxy patients ( Figures 1b and 2b ). The percentage of IgM memory B cells in the heterotaxy patients was only 1.8 ± 2.1%, significantly lower than the percentages in the CHD groups: (3.9 ± 3.2 and 5.1 ± 4.7% in the complex and simple CHD groups, respectively) ( Table 1 ). Again, age, sex, and oxygen saturation were not associated with the IgM memory B-cell count in the heterotaxy or control groups.
Low IgM Memory B-Cell Count and the Risk of Community-Acquired SBI in Heterotaxy
In the heterotaxy group, 76% of the patients had a low memory B-cell percentage (defined as < 10%), and 41% had a low IgM memory B-cell percentage (defined as <1%) (15). Table 2 presents a comparison of the clinical data on the patients with low and normal IgM memory B-cell percentages. Age, sex, and oxygen saturation did not differ significantly between the groups. Although the imaging studies detected no spleen in 69% of the heterotaxy patients, some of the patients without spleen identified in the imaging studies exhibited normal IgM memory B-cell counts. Comparing patients with and without a spleen according to the imaging studies, the memory B-cell percentage (10.0 ± 6.3 vs. 7.4 ± 7.6%, P = 0.273) and IgM memory B-cell percentage (2.1 ± 1.2 vs. 1.7 ± 2.4%, P = 0.533) were lower in those without a spleen, but this difference was not statistically significant ( Figure 3 ).
Memory B and IgM memory B cell percentage in heterotaxy patients with or without spleen by imaging study. (a) The memory B-cell percentage and (b) IgM memory B-cell percentage between those without and with spleen visible in an imaging study. Although the memory B cell and IgM memory B-cell percentage are both a little lower in those without spleen in an imaging study, the difference is not significant.
After setting a cutoff point at 1% of CD22+ cells, we determined that the community-acquired SBI rate was 31.6% in the heterotaxy patients with a low (<1 %) IgM memory B-cell percentage. By contrast, in those with a IgM memory B-cell proportion of > 1%, the community-acquired SBI rate was 3.8% (P = 0.031). The culture-positive SBI rate was also higher in those with a low IgM memory B-cell percentage compared with those with a high percentage, although not significantly (15.8 vs. 0%, P = 0.064). Of the three patients with culture-positive SBI, one was an 11-y-old boy who had experienced two episodes of community-acquired SBI (one episode was pneumococcal sepsis at age 6; the other episode was Citrobacter freundii sepsis at 3 mo of age). Another patient was a boy aged 5.3 y who contracted pneumococcal meningitis at 5 y of age. Another patient was a boy aged 10 who had a brain abscess at 7.5 y of age. All three of these patients had a low IgM memory B-cell percentage (< 1%), and the memory B cells and IgM memory B cells were nearly undetectable in them (0–1.1% of memory B cells and 0–0.15% of IgM memory B cells) ( Figures 1a , b and 2c , d ). By using multivariate logistic regression including age, sex, oxygen saturation, imaging-determined spleen status, and low IgM memory B-cell percentage, we found that the presence of a low IgM memory B-cell percentage was the only significant factor related to community-acquired SBI (odds ratio: 12.0, 95% confidence interval: 1.3–110, P = 0.028).
Discussion
Using the proposed diagnostic tool, we found that (i) the memory B cell and IgM memory B-cell percentages were significantly lower in the heterotaxy group than in the control groups; and (ii) In the heterotaxy patients, the memory B cell and IgM memory B-cell percentages were lower in those with previously documented community-acquired SBI and culture-positive SBI. A low IgM memory, B-cell percentage was significantly associated with community-acquired SBI.
Measurement of Splenic Immunologic Function
Although the spleen is a vital immunologic organ, assessing its function is still difficult. Traditionally, the Howell–Jolly body count is considered an indicator of markedly decreased splenic function (17). However, the clinical implication is limited by suboptimal sensitivity and a lack of objective counting (18). A newly introduced hematology method involves pit count of circulating erythrocytes by using interference contrast microscopy (13). This method has been suggested to be a more sensitive marker than the Howell–Jolly body count in detecting splenic function; however, the involved technical complexity limits its application in clinical practice (13,19). Recently, the percentages of the memory B cell and IgM memory B-cell counts in the peripheral blood have been shown to correlate favorably with splenic function (15,16,20). The IgM memory B-cell count decreased in patients with hyposplenism, including patients with common variable immunodeficiency disease and inflammatory bowel disease (16). In addition, a high correlation was found between the pitted red cell value and IgM memory B-cell count (16). These data support the use of memory B and IgM memory B-cell counts as indicators of splenic function. However, their clinical use in young patients with heterotaxy syndrome is still limited (21). In the present study, we found considerably lower memory B cell and IgM memory B-cell percentages in the heterotaxy patients compared with the control complex and simple CHD groups.
Low IgM Memory B-Cell Percentage in Heterotaxy Patients Is Correlated With Community-Acquired SBI
Studies on SBI in patients with heterotaxy syndrome are limited (8). In our previous study on 95 heterotaxy patients, we found that community-acquired culture-positive SBI was common in heterotaxy patients with a 5-y cumulative infection rate of 14.5%, but not in complex CHD patients (5-y cumulative infection rate of 0%) (6). Although splenic immune function was generally lower in the RAI patients, its rate varied. In that study, we determined that repeat SBI episodes in the same patient are common, suggesting that a variation of individual susceptibility to SBI exists among RAI patients (6). In addition to individual variation, splenic function may change with age. The SBI rate has been reported to be higher in the first 2 y of life and to decrease gradually with age (6,11). In our previous study, the annual infection rate was 7.1% in the first 2 y of life, 3.3% between the ages of 2 and 5 y, and 1.5% after the age of 5 y (6). Other lymphoid organs, such as the lymph nodes and enteric lymphoid tissue, may substitute for splenic function with age and reduce the risk of SBI in some patients (11). Because of individual variations and changes of immune function over time, measuring splenic function is essential in identifying patients at high-risk of SBI.
Spleen status can be assessed in imaging studies, but this is not effective in identifying patients at risk of infection from hyposplenism (22). This is because some heterotaxy patients may still have accessory splenic tissue with variable splenic immune function (i.e., atrial visceral situs discrepancy) (6,7), or have abnormal splenic function despite the presence of splenic tissue (e.g., LAI patients) (6,8,21). Directly measuring splenic immune function may be informative in identifying patients at risk of infection. In the current study, we observed that the memory B cell and IgM memory B-cell percentages in heterotaxy patients were closely correlated with the occurrence of SBI. This correlation was stronger than that obtained using imaging studies to determine spleen status. In addition, in each of the three patients with a history of culture-positive SBI, both the memory B and IgM memory B-cell percentages were low. A low IgM memory, B-cell percentage (<1%) was the only factor significantly correlated with community-acquired SBI. These findings indicate that the memory B and IgM memory B-cell percentages are more accurate indicators of hyposplenism and SBI risk in RAI patients than imaging studies.
The current hyposplenia treatment guidelines in United States and Europe recommend long-term antibiotic prophylaxis for high-risk patients (21,22,23). However, the suggested treatment duration is controversial (23), ranging from 2 y to lifelong, and the antibiotic prophylaxis recommendation has often been questioned. First, the guideline does not consider individual disease differences (24). The only evidence of antibiotic prophylaxis in children is from sickle cell disease cases (23). Second, the guideline does not consider antibiotic resistance, which is an emerging problem because of antibiotic overuse (25,26). Because of the high resistance rate of pneumococcus, antibiotic prophylaxis policy in Taiwan is emergency use (in other words, use of antibiotics only if fever occurs or with suspicion of infection). The rationale for this policy is to reduce pneumococcus resistance; however, it may have increased the SBI rate in our cohort. Therefore, the new markers proposed in our study may select real high-risk patients and provide a more appropriate antibiotic prophylaxis decision in heterotaxy patients. Whether these markers should be incorporated into the guidelines for antibiotic prophylaxis treatment of RAI patients requires a long-term follow-up study.
The vaccine for the encapsulated bacteria is a critical part of preventing SBI in heterotaxy patients (22). One-third of the patients in our study did not receive a pneumococcal vaccine, but most of them received the Hemophilus influenza vaccine. The reason for the low pneumococcal vaccination rate is related to government subsidies. This low vaccination rate may have interfered with our study results, inflating the SBI rate. However, in our study, the two patients with documented pneumococcal sepsis and meningitis had previous pneumococcal vaccination (one was infected with a nonvaccine strain and the other received only the polyvalent polysaccharide pneumococcal vaccine). Furthermore, the unresponsiveness to pneumococcal conjugate vaccine is also a problem in hyposplenism patients (22). Whether incorporating these markers can identify patients who are unresponsive to pneumococcal conjugate vaccine requires further study.
Confounders of Memory B Cell and IgM Memory B-Cell Measurements
Very young age (infancy) is a possible confounder in applying the memory B cell and IgM memory B-cell counts for predicting patients at high risk of SBI. When the human body is exposed to foreign polysaccharide antigens, the splenic marginal zone germinal center B cell responds. After proliferation, somatic mutation, selection, and class switching, the final products of the germinal center reaction are high-affinity memory B cells and IgM memory B cells. Because the foreign antigen exposure is still inadequate in the infant period, memory B cell and IgM memory B-cell production is generally low and often undetectable, even in normal infants (15), which is why we enrolled only patients aged at least 1 y.
We found no association of memory B cell or IgM memory B-cell percentages with oxygen saturation or age, which have been suggested as confounders (15,27). Pearson et al. reported two cases of the transposition of the great arteries in patients with hypoxia and both had hyposplenism; however, these cases were questioned by William et al., who theorized that the two cases should be diagnosed as heterotaxy syndrome. In the present study, we found that the patients in the RAI group had a significantly lower IgM memory B-cell percentage than that of those in the complex CHD group despite having similar oxygen saturation. This finding ruled out oxygen saturation interfering in the IgM memory B-cell analysis. A positive correlation between age and IgM memory B-cell count was proposed by Kruetzmann et al., but this relationship was more prominent in patients younger than 1 y of age (15). Because the current study was limited to patients aged older than 1 y, we observed no association between age and IgM memory B-cell count.
In patients with protein-losing enteropathy, lymphopenia is a common characteristic caused by a loss in stool (28,29). Therefore, whether the low memory B cell and IgM memory B-cell measurements reflect an increased susceptibility to SBI is still uncertain in this clinical scenario, and must be explored in future studies.
Study Limitation
Because heterotaxy syndrome is a rare disease, relatively few patients could be enrolled in our study cohort. Only seven community-acquired SBI patients and three community-acquired culture-positive SBI patients were enrolled. However, we still found a low IgM memory B-cell percentage to be the only significant factor correlated with community-acquired SBI.
Because memory B cells and IgM memory B cells are often few and undetectable in infancy (15), we could not apply this newly introduced hematology method to measure splenic function in infants. This may limit the clinical application of the current method because SBI often occurs during infancy.
Conclusion
Memory B cell and IgM memory B-cell percentages are lower in heterotaxy patients than in complex and simple CHD patients. In heterotaxy patients, the presence of a low (< 1%) IgM memory B-cell percentage is correlated with community-acquired SBI episodes.
Methods
Subjects
We enrolled all patients aged more than 1 y who had been diagnosed with heterotaxy syndrome and received follow-up care at National Taiwan University Hospital, which is the referral center for complex CHD in Taiwan, between January 2010 and December 2012. We performed our study in accordance with the regulations of the National Taiwan University Hospital institutional review board, which were in accordance with the Helsinki Declaration. All patients signed the informed consent before entering study (or signed by parents). We reviewed the charts of the patients and recorded previously documented SBI events including community-acquired episodes and nosocomial infections. The diagnostic criteria of RAI and LAI included bilateral atrial appendage morphology belonging to the right atrium and left atrium, accompanied by bilateral symmetric tracheobronchial trees, as confirmed during surgery or by using computed tomography imaging. Spleen status was determined using either computed tomography or abdominal sonography. The definition of SBI included (i) a positive blood culture combined with clinical signs of sepsis and an elevated white blood cell count or inflammation markers such as C-reactive protein; (ii) a brain abscess, empyema, or other abscess formation requiring drainage; and (iii) bacterial meningitis, infective endocarditis, pyelonephritis, or bacterial pneumonia (including lobar pneumonia or pneumonia with elevated inflammation markers). Patients with SBI and a positive culture result (including blood, cerebral spinal fluid, or an abscess) were defined as culture-positive SBI. Community-acquired SBI was defined as SBI with presentation or a positive culture result within 48 h after admission and no interventions or hospitalizations within the week before admission. The control group in our study comprised 67 patients with complex CHD and 66 patients with simple CHD (atrial septal defect in 32 patients, patent ductus arteriosus in 19 patients, and ventricular septal defect in 15 patients), all without genetic syndromes. The definition of complex CHD in our study was CHD requiring single ventricle physiology surgery; among the types of CHD studied were tricuspid atresia (11 patients), double-inlet left ventricle (10 patients), hypoplastic left heart syndrome (8 patients), double-outlet right ventricle (8 patients), l-transposition of the great arteries (8 patients), double inlet double outlet ventricle (7 patients), mitral atresia with double outlet right ventricle (5 patients), pulmonary atresia with intact ventricular septum (5 patients), and other (5 patients).
Protein-losing enteropathy was found in five patients after Fontan-type operations (four in the complex CHD group and one in the heterotaxy group). Patients with protein-losing enteropathy may have a very low lymphocyte count; thus, memory B cell and IgM memory B cell counting may be impaired. Therefore, these five patients were excluded.
Flow Cytometry
After receiving written informed consent from the patients or their parents, 5 ml of fresh blood was sampled from peripheral blood or during cardiac catheterization. Mononuclear cells were isolated from heparinized blood by using Ficoll-Paque Plus (GE Healthcare Bio-Sciences, Uppsala, Sweden). The blood mononuclear cells were stained with the appropriate antibody combinations of fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll protein complex (Per-CP), and allophycocyanin. Monoclonal antibodies HIB19 (anti-CD19), HIB22 (anti-CD22), M-T271 (anti-CD27), G20–127 (anti-IgM), and IA6–2 (anti-IgD) were obtained from Becton Dickinson (San Jose, CA). Dead cells were excluded from analysis by using forward and side scatter gating. All analyses were performed on a four-color flow cytometry FACSCalibur (Becton Dickinson) interfaced with a Macintosh CellQuest computer program. The CD19+ cell, identified as B lymphocyte, was gated for subsequent analysis. The percentage of memory B cells was determined using flow cytometry-based immunophenotyping of the surface markers CD22+ and CD27+ in the CD22+ cells. IgM memory B cells were identified according to the percentage of immunophenotyping of the surface markers of the CD22+, CD27+, IgM+, and IgD+ cells in the CD22+ cells.
Statistical Analysis
SPSS version 15.0 (SPSS, Chicago, IL) was used to perform statistical analysis. We used the Student t-test or one-way analysis of variance to compare data with normal distribution. We used a nonparametric method with the Mann–Whitney or Kruskal–Wallis test to compare data with skewed distribution. Categorical data were examined using a chi-square test and Fischer’s exact test. The data were presented as mean ± SD; P < 0.05 was considered statistically significant.
Statement of Financial Support
This work was supported by National Science Council in Taiwan (grant 101-2314-B-002-034-MY3).
Disclosure
None of the authors have conflicts of interest or financial relationships relevant to this article to disclose.
References
Lin JH, Chang CI, Wang JK, et al. Intrauterine diagnosis of heterotaxy syndrome. Am Heart J 2002;143:1002–8.
Yan YL, Tan KB, Yeo GS. Right atrial isomerism: preponderance in Asian fetuses. Using the stomach-distance ratio as a possible diagnostic tool for prediction of right atrial isomerism. Ann Acad Med Singapore 2008;37:906–12.
Chiu IS, How SW, Wang JK, et al. Clinical implications of atrial isomerism. Br Heart J 1988;60:72–7.
Taketazu M, Lougheed J, Yoo SJ, Lim JS, Hornberger LK. Spectrum of cardiovascular disease, accuracy of diagnosis, and outcome in fetal heterotaxy syndrome. Am J Cardiol 2006;97:720–4.
Wu MH, Wang JK, Lue HC. Sudden death in patients with right isomerism (asplenism) after palliation. J Pediatr 2002;140:93–6.
Chiu SN, Shao PL, Wang JK, et al. Severe bacterial infection in patients with heterotaxy syndrome. J Pediatr 2014;164:99–104.e1.
Ticho BS, Goldstein AM, Van Praagh R. Extracardiac anomalies in the heterotaxy syndromes with focus on anomalies of midline-associated structures. Am J Cardiol 2000;85:729–34.
Prendiville TW, Barton LL, Thompson WR, Fink DL, Holmes KW. Heterotaxy syndrome: defining contemporary disease trends. Pediatr Cardiol 2010;31:1052–8.
Park MK. Cyanotic congenital heart defect: heterotaxia (atrial isomerism, splenic syndromes). In: Park MK, ed. Pediatric Cardiology for Practitioners. 5th edn. St. Louis, MO: Mosby; 2008.
Wu MH, Wang JK, Lin JL, et al. Supraventricular tachycardia in patients with right atrial isomerism. J Am Coll Cardiol 1998;32:773–9.
William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology 2007;12:1–13.
Evans DI. Postsplenectomy sepsis 10 years or more after operation. J Clin Pathol 1985;38:309–11.
Pearson HA, Johnston D, Smith KA, Touloukian RJ. The born-again spleen. Return of splenic function after splenectomy for trauma. N Engl J Med 1978;298:1389–92.
Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004;104:3647–54.
Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 2003;197:939–45.
Di Sabatino A, Rosado MM, Ciccocioppo R, et al. Depletion of immunoglobulin M memory B cells is associated with splenic hypofunction in inflammatory bowel disease. Am J Gastroenterol 2005;100:1788–95.
Lipson RL, Bayrd ED, Watkins CH. The postsplenectomy blood picture. Am J Clin Pathol 1959;32:526–32.
Corazza GR, Ginaldi L, Zoli G, et al. Howell-Jolly body counting as a measure of splenic function. A reassessment. Clin Lab Haematol 1990;12:269–75.
Corazza GR, Bullen AW, Hall R, Robinson PJ, Losowsky MS. Simple method of assessing splenic function in coeliac disease. Clin Sci (Lond) 1981;60:109–13.
Wasserstrom H, Bussel J, Lim LC, Cunningham-Rundles C. Memory B cells and pneumococcal antibody after splenectomy. J Immunol 2008;181:3684–9.
William BM, Thawani N, Sae-Tia S, Corazza GR. Hyposplenism: a comprehensive review. Part II: clinical manifestations, diagnosis, and management. Hematology 2007;12:89–98.
Davies JM, Lewis MP, Wimperis J, Rafi I, Ladhani S, Bolton-Maggs PH ; British Committee for Standards in Haematology. Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: prepared on behalf of the British Committee for Standards in Haematology by a working party of the Haemato-Oncology task force. Br J Haematol 2011;155:308–17.
Spelman D, Buttery J, Daley A, et al.; Australasian Society for Infectious Diseases. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J 2008;38:349–56.
Butler C, Kinnersley P. Managing patients with an absent or dysfunctional spleen. Is there evidence to show that daily antibiotic treatment is best? BMJ 1996;312:1360–1.
Lambert HP. Managing patients with an absent or dysfunctional spleen. Guidelines do not discuss resistance to antibiotics among pneumococci. BMJ 1996;312:1361.
Hsieh YC, Huang YC, Lin HC, et al. Characterization of invasive isolates of Streptococcus pneumoniae among Taiwanese children. Clin Microbiol Infect 2009;15:991–6.
Pearson HA, Schiebler GL, Spencer RP. Functional hyposplenia in cyanotic congenital heart disease. Pediatrics 1971;48:277–80.
Chakrabarti S, Keeton BR, Salmon AP, Vettukattil JJ. Acquired combined immunodeficiency associated with protein losing enteropathy complicating Fontan operation. Heart 2003;89:1130–1.
Cheung YF, Tsang HY, Kwok JS. Immunologic profile of patients with protein-losing enteropathy complicating congenital heart disease. Pediatr Cardiol 2002;23:587–93.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiu, SN., Shao, PL., Wang, JK. et al. Low immunoglobulin M memory B-cell percentage in patients with heterotaxy syndrome correlates with the risk of severe bacterial infection. Pediatr Res 79, 271–277 (2016). https://doi.org/10.1038/pr.2015.221
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2015.221
This article is cited by
-
A multi-disciplinary, comprehensive approach to management of children with heterotaxy
Orphanet Journal of Rare Diseases (2022)
-
Pneumococcal vaccination and efficacy in patients with heterotaxy syndrome
Pediatric Research (2017)