Abstract
Background:
Significant hemodynamic changes occur immediately after birth in preterm infants. Amplitude-integrated electroencephalography (aEEG) provides a method of assessing brain activity in sick neonates; however, the relationship among systemic blood flow, blood pressure (BP), and aEEG is not clear.
Methods:
Quantitative measures of aEEG continuity and amplitude were correlated with superior vena cava (SVC) flow, right-ventricular output (RVO), and BP at 12, 24, and 48 h in 92 infants born at <29 wk gestation.
Results:
SVC flow, RVO, BP, aEEG amplitude, and EEG continuity all increased from 12 to 48 h. SVC flow at 12 h, but not 24 or 48 h, was significantly associated with aEEG amplitude after adjustment for gestational age (GA) and severity of illness markers (r2 = 0.21, P = 0.004). RVO and BP showed less consistent associations with aEEG parameters. Infants receiving inotropes at 12 h, including those in whom cardiovascular parameters had normalized, had significantly lower aEEG amplitude (P < 0.01) and EEG continuity at the 10, 25, and 50 μV levels (P < 0.01) at 12, 24, and 48 h than neonates who were not receiving inotropes.
Conclusion:
aEEG measurements in the first 48 h of life are related to SVC flow and treatment with inotropes at 12 h of life in extremely preterm infants.
Similar content being viewed by others
Main
Amplitude-integrated electroencephalography (aEEG) is increasingly being used to assess encephalopathic full-term infants, in whom persisting EEG discontinuity is associated with adverse neurodevelopmental outcome (1,2). The role of aEEG in preterm neonates is less certain. We have previously shown an association between low aEEG continuity and poor short-term outcome in extremely premature neonates (3); however, the acute effect of neonatal interventions and physiological parameters such as systemic blood flow and blood pressure (BP) on aEEG is still not clear. Interventional procedures undertaken in the first few days of life have been shown to affect aEEG recording, possibly through alteration in cerebral blood flow (CBF). Hellström-Westas et al. (4) investigated the effect of surfactant treatment on continuous aEEG recordings and found a short (<10 min) but significant decrease in cerebral activity in almost all the neonates immediately after the surfactant instillation, despite improved pulmonary function. Although a number of studies have assessed the relationship between hemodynamic parameters and CBF in neonates (5,6,7,8,9,10), few studies have assessed the relationship between hemodynamic parameters and aEEG in the first 48 h after birth (11,12).
Significant hemodynamic changes occur immediately after birth; these changes may affect cerebral circulation, neuronal activity, and neurodevelopmental outcome. A number of measures can be used to assess hemodynamic status and cardiac output in the neonate, including BP, right-ventricular output (RVO), left-ventricular output, and superior vena cava (SVC) flow (13,14). Low SVC flow in the first 24 h after birth has been associated with periventricular hemorrhage (15) and adverse neurodevelopmental outcome at 3 y of age (16) and may be a stronger predictor of adverse outcome than BP (16).
The aEEG provides continuous monitoring of cerebral activity and can be used in most preterm infants (17). There is a paucity of evidence looking at the effect of systemic blood flow on the electrocortical activity of extremely preterm infants as measured by aEEG (3,10,12) and EEG (11) in the first 48 h of life. The little information that is available is conflicting: some studies have found an association between abnormal aEEG activity and low cardiac output, specifically low RVO (12,18); other studies do not show an association (3,10,11). The aim of this study was to assess the associations between SVC flow, RVO, BP, and quantitative aEEG parameters in the first 48 h of life in infants born at 24–28 wk gestation.
Results
A total of 92 infants were enrolled in the study: 28 infants born at 24–25 wk gestation and 64 infants born at 26–28 wk gestation. Six neonates died between 24 and 48 h. Satisfactory aEEG trace was available for 90 infants at 12 h, 88 infants at 24 h, and 75 infants at 48 h. Complete cardiovascular data were available for 90, 87, and 50 infants at 12, 24, and 48 h, respectively. Perinatal and clinical characteristics are shown in Table 1 . Only five infants were receiving sedation (morphine) and none were treated with muscle relaxants or anticonvulsants during the course of the study. At the time of initial assessment at 12 h, 12 (13%) infants had BP <25 mmHg, 12 (13%) infants had RVO ≤120 ml/kg/min, and 18 (20%) infants had SVC flow ≤45 ml/kg/min. Twenty-seven infants (29%) had one or more of these cardiovascular parameters below the normal range at 12 h of age (abnormal cardiovascular hemodynamics). At 24 h, only 9 (10%) infants had cardiovascular parameters below the normal range. Of the 32 infants who received dopamine/dobutamine for low BP or poor perfusion, 20 (62%) infants had commenced inotrope treatment before the first study assessment at 12 h. Eleven of these (55%) continued to have low SVC flow or low BP at 12 h, despite treatment with inotropes. Twelve infants commenced inotrope treatment after 12 h.
The mean blood flow (SVC and RVO), BP, aEEG amplitude, and EEG continuity all increased from 12 to 48 h (Friedman test, P < 0.001 for all analyses) ( Tables 2 and 3 ).
Hemodynamic Parameters and aEEG Amplitude and Continuity
On univariate regression analysis, there were significant correlations between blood flow (SVC and RVO) and BP measurements at 12 h and aEEG minimum and mean amplitude at 12 h ( Figure 1 ). Associations were also found between aEEG parameters at 12 h, Apgar score at 1 min, and mechanical ventilation at 12 h. After adjustment for these factors and for gestational age (GA) in multiple regression analysis, SVC flow remained significantly associated with aEEG minimum, mean, and maximum amplitude at 12 h ( Table 4 ), with a more variable and weaker association seen between aEEG amplitude and RVO and BP measures. There was no significant correlation between blood flow (SVC and RVO) and BP measurements at 24 and 48 h and aEEG amplitude at 24 and 48 h, respectively ( Table 4 ). After adjustment for GA, hemodynamic measures did not correlate with any of the EEG continuity measures.
Correlation of minimum and mean amplitude at 12 h with (a,b) superior vena cava (SVC) flow, (c,d) right-ventricular output (RVO), and (e,f) mean blood pressure (BP) at 12 h. Vertical hashed line in a and b indicates low SVC flow (≤45 ml/kg/min), in c and d indicates low RVO (≤120 ml/kg/min), and in e and f indicates low BP (mean BP <25 mmHg).
Subgroup Analysis: Infants With Low SVC Flow and Low BP
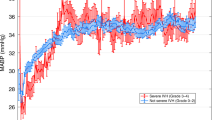
Infants with low SVC flows (≤45 ml/kg/min) at 12 h, had significantly lower minimum amplitude at 12 h (3.55 μV vs. 3.90 μV, P = 0.033), with a trend toward a slower rate of increase in aEEG amplitude by 48 h as compared with infants with normal SVC flows ( Figure 2 ). There were no statistically significant associations between low SVC flow and EEG continuity or between low mean BP (<25 mm Hg) and any aEEG or EEG measures.
Median amplitude-integrated electroencephalography amplitudes at 12, 24, and 48 h for neonates with low (≤45 ml/kg/min) and normal (>45 ml/kg/min) superior vena cava flows. *P < 0.05 for minimum amplitude. Solid black line: maximum amplitude; dashed line: mean amplitude; dotted line: minimum amplitude.
Subgroup Analysis: Infants Receiving Inotropes for Clinical Management
Infants who commenced inotropes treatment before 12 h of age for clinical management of low BP or poor perfusion had significantly lower mean, minimum, and maximum aEEG amplitude ( Figure 3 ) and significantly lower EEG continuity at the 10, 25, and 50 μV levels ( Figure 4 ) at 12, 24, and 48 h as compared with the neonates who were not receiving inotropes at 12 h (P < 0.01 for all ANOVAs). Significant differences were seen between neonates receiving inotropes at 12 h as compared with those who were not receiving inotropes, both for the subgroup group whose cardiovascular parameters had normalized (P < 0.05 for mean and median aEEG amplitude and EEG continuity at the 10 μV level at 12 h) and for those with continuing abnormal cardiovascular hemodynamic parameters (P < 0.01 for all measures of aEEG amplitude and EEG continuity at 12 h) ( Figures 3 and 4 ).
Group minimum (open triangles), mean (solid diamonds), and maximum (open circles) amplitude-integrated electroencephalography (aEEG) amplitudes at 12 h for neonates treated with and without inotropes. *P < 0.05 as compared with no inotropes, normal CVS; **P < 0.05 as compared with no inotrope, abnormal CVS. CVS, cardiovascular hemodynamics.
Median group values for percentage electroencephalography (EEG) continuity at 12 h for neonates treated with and without inotropes. Black bars, 50 μV; white bars, 25 μV; gray bars, 10 μV; hatched bars, <10 μV.*P < 0.05 as compared with no inotropes, normal CVS. **P < 0.05 as compared with no inotrope, abnormal CVS. CVS, cardiovascular hemodynamics.
Discussion
In this study of 92 extremely preterm infants (<29 wk GA), we found a significant association between SVC flow and aEEG amplitude at 12 h of life, but not at 24 or 48 h, after adjustment for GA and severity of illness markers. The association was no longer present at 24 or 48 h. Infants with low SVC flow (≤45 ml/kg/min) had significantly lower minimum aEEG amplitude at 12 h and a trend toward a slower increase in amplitude over the first 48 h of life as compared with those with normal SVC flow at 12 h. Associations between RVO, BP, and aEEG measures were weaker and less consistent than for SVC flow. Infants receiving inotropes for clinical management of low BP or poor perfusion before 12 h of age had significantly lower aEEG amplitude and continuity at 12, 24, and 48 h, with differences persisting even among those neonates whose cardiovascular parameters were within the normal range at 12 h.
Significant hemodynamic changes that occur immediately after birth may affect cerebral circulation, neuronal activity, and neurodevelopmental outcome (15,16). Consistent with the study by Osborn et al. (18), infants in our study had decreased systemic blood flow and BP at 12 h as compared with later assessments, with 29% of the cohort identified as having one or more cardiovascular parameters below the normal range at 12 h. At the time of this nadir in blood flow, there was a significant correlation on linear regression analysis between cardiovascular factors and aEEG amplitude. With increasing SVC, RVO, and BP values at 24 and 48 h, the correlation between cardiovascular factors and aEEG was no longer present.
The association seen in this study between aEEG amplitude and cardiovascular measures at 12 h may represent a causal pathway, with low systemic blood flow, low BP, and poor autoregulation in the first 12 h of life resulting in low CBF and associated decrease in aEEG activity. Alternatively, the association may represent an indirect link, with infants experiencing low blood flow, low BP, and low aEEG amplitude due to a common underlying problem. For example, infants may have experienced an earlier physiological insult, resulting in both decreased neuronal activity and poor myocardial function.
Electroencephalographic activity is known to be associated with CBF, with EEG depression in adults occurring with blood flow <10 ml/100 g/min (19), although the reduction in blood flow required to cause EEG depression in neonates is less certain (20). The associations between systemic blood flow/BP and both CBF and EEG activity in neonates are more complex and are influenced not only by blood supply available to the brain but also by autoregulation mechanisms acting within the brain. Autoregulation may be inherently poorly developed in extremely premature infants, or may be impaired by an early hypotensive or ischemic insult (21).
Several studies have assessed the relationship between BP or systemic blood flow and CBF (5,6,7,8,9,10), with a range of conclusions. Few previous studies have assessed the relationships between hemodynamic parameters in neonates and EEG (11,12) in preterm neonates in the first 48 h of life. A study by West et al. (12), which assessed the relationships between RVO, SVC flow, BP, aEEG amplitude, and EEG continuity in the first 48 h of life in 40 infants born at <31 wk gestation, found similar associations between BP, RVO, and aEEG amplitude at 12 h, with only limited associations beyond 12 h. In the study by West et al., SVC flow was not associated with aEEG parameters. Median SVC flows at 12 h reported in the study by West et al. were higher than in our study and may reflect a more mature cohort or fewer critically ill neonates.
A study by Victor et al. (11), which assessed 40 preterm infants born at <30 wk gestation in the first 4 d of life, did not identify any statistically significant relationship between left-ventricular output, RVO, or BP with cerebral electrical activity (measured using digital EEG) or cerebral fraction oxygen extraction. Qualitative assessment of the EEG was normal in all infants with BP >30 mmHg, even when cardiac output (left-ventricular output and RVO) was low. However, four neonates with critically low BP (mean BP <23 mmHg) had qualitatively abnormal EEG records, with suppression or prolonged discontinuity of the EEG consistent with a similar finding in our study of decreased minimum aEEG amplitude associated with low SVC flow.
Despite the association between hemodynamic variables and CBF or EEG reported by some researchers, studies providing treatment to increase BP or systemic blood flow, including albumin infusion (7,10), closure of patent ductus arteriosus, (22) or treatment with inotropes (7) have failed to show an effect of increasing hemodynamic output on CBF or EEG activity. These findings may be due to two separate mechanisms. In neonates with normal BP and cardiac output before treatment it has been hypothesized that the lack of change in the EEG is related to intact autoregulation (9), whereas in neonates who are unwell with hypotension or poor cardiac output before treatment it is hypothesized that the lack of improvement in the EEG is related to poor reversibility of neural dysfunction (10).
Our study showed associations between the use of inotropes at 12 h and persistently decreased aEEG amplitude and EEG continuity in the first 48 h of life, even in those infants in whom cardiovascular measures had improved into the normal range. Although we cannot exclude a direct effect of inotropes on CBF or neuronal activity, we considered the use of inotropes to be a marker for neonates who have experienced an earlier period of poor perfusion, which in turn may have been associated with hypoxic/ischemic injury and/or impairment of autoregulation. Infants treated with inotropes who had persistently abnormal cardiovascular parameters were found to have more severe depression of the aEEG amplitude and EEG continuity, consistent with a more prolonged period of low perfusion. However, even those whose hemodynamic parameters (SVC flow, RVO, and BP) had normalized after treatment with inotropes had depression of aEEG amplitude and EEG continuity as compared with neonates who had not required inotrope treatment before 12 h of life, indicating that aEEG activity in the first 48 h of life may be more strongly associated with earlier ischemic insults than with contemporaneous hemodynamic parameters.
Strengths and Limitations
This study is the largest cohort to date to provide detailed cardiovascular and aEEG outcomes in extremely premature neonates in the first 48 h of life. The use of SVC flow as a measure of upper-body perfusion provides an indirect measure of cerebral perfusion, which is less influenced by cardiac shunts than RVO or left-ventricular output measures.
Limitations of this study include lack of direct measurement of CBF. In future studies, use of cerebral Doppler flows or near-infrared spectroscopy may provide a clearer understanding of the relationship between CBF and aEEG. Measurement of CO2 levels and other factors known to reduce CBF would also have enhanced this study.
Conclusion
aEEG amplitude at 12 h is significantly associated with SVC flow in extremely premature neonates after adjusting for GA and severity of illness markers. This association was no longer present at 24 or 48 h.
Neonates treated with inotropes at 12 h for clinical management of low BP or poor perfusion had persistently decreased aEEG amplitude and EEG continuity in the first 48 h of life, including in those for whom cardiovascular measures had normalized.
Methods
Subjects
Infants with GA <29 wk admitted to two tertiary-level neonatal intensive care units at Royal North Shore Hospital and Royal Prince Alfred Hospital (Sydney, Australia), were recruited to this prospectively performed cohort study between January 2004 and August 2008. The study was approved by the human research ethics committee and the clinical trials committee of both the hospitals. Written informed parental consent was obtained for all neonates. For the majority of infants studied, consent was obtained before birth. Perinatal characteristics recorded included GA at birth, birth weight, mode of delivery, cord blood pH and base excess, and Apgar scores at 1 and 5 min. Clinical management during the study period was documented, including mechanical ventilation, use of high-frequency ventilation, and treatment with sedatives, anticonvulsants, and inotropes (dopamine or dobutamine). Treatment with inotropes was at the discretion of the attending clinician but was generally given for low BP (mean BP <25 mmHg) or clinical and cardiac ultrasound signs of poor perfusion (13). Major adverse clinical outcomes, including worst grade of intraventricular hemorrhage (reported by a radiologist) and neonatal survival, were recorded.
aEEG
Two-channel aEEG traces were recorded continuously from 10 to 48 h of life with the Brainz Instruments BRM2 monitor (Brainz Instruments, Auckland, New Zealand), using hydrogel electrodes, with standard electrode placement at the C3, P3, C4, and P4 regions (International 10–20 system). The transformation of the EEG signal into the amplitude-integrated trace has been described elsewhere (23,24). The EEG signal is filtered, rectified, and amplitude-integrated. The final result is plotted on a semi-logarithmic scale (linear 0–10 μV, logarithmic 10–100 µV) display of the peak-to-peak amplitude values of EEG, to emphasize the lower voltage activity. The bandwidth of the aEEG reflects variation in minimum and maximum amplitudes. The aEEG tracing is viewed on a highly compressed time scale, a rate of 6 cm/h. A full minute of EEG is represented by only a single millimeter of aEEG display. Analysis is performed using specific computer software to obtain quantitative measures.
All aEEG recordings were obtained by two investigators (M.P. and D.S.). These recordings were analyzed for three 2-h epochs at 12–14 h, 24–26 h, and 46–48 h. Data were analyzed when there was at least 60 min of satisfactory recording within the 2-h epoch, with impedance <10 kOhm per electrode pair. Cross-cerebral P3–P4 aEEG recordings were analyzed to provide comparison with single-channel aEEG monitors such as the cerebral function monitor, as these measures were functionally equivalent to the cerebral function monitor (25).
Quantitative analysis of the aEEG. Quantitative measurements of cross-cerebral P3–P4 recordings were analyzed off-line using the Brainz Analyze Research version 1.5 software (Brainz Instruments) by a blinded observer (J.R.B.) after completion of all cardiovascular measurements. The Brainz Analyze software exports the raw data and calculates 1-min average values for the maximum, minimum, and mean amplitudes of the aEEG traces. The aEEG data were assessed every 2 s, then averaged, and recorded at 1-min intervals (24).
EEG continuity. Quantitative EEG continuity measures were determined for each 2-h epoch as the percentage of time during which the amplitude of the raw EEG was above a predetermined threshold (10, 25, and 50 μV) (24).
aEEG amplitude. The mean ± SD of the maximum, mean, and minimum aEEG amplitude was calculated for each 2-h epoch.
BP
BP monitoring was performed using a Nova-dome pressure transducer (Micromed, Woking, UK) attached to an indwelling arterial catheter. Noninvasive BP was measured by oscillometry (GE Healthcare, Waukesha, WI) measured hourly by automatic recording. Low BP was defined as a mean BP recording <25 mmHg.
Cardiac Output
An Acuson 128/XP Aspen (Acuson, Mountain View, CA) or GE Vivid 7 (GE Healthcare, Horton, Norway) ultrasound scanner was used with a 7 MHz vector array transducer incorporating color and pulsed-wave Doppler. Echocardiographic ultrasound was performed at 12, 24, and 48 h of age (± 2 h) by the researchers (M.P. and D.S.) before any aEEG analysis. Structural normality of the heart was established at initial scan. Hemodynamic parameters measured by echocardiogram were SVC flow, RVO, and patent ductus arteriosus diameter (15). The techniques and methods for SVC flow and RVO have been described previously (13,26). Low SVC flow was defined as an SVC flow ≤45 ml/kg/min (14), and low RVO was defined as an RVO ≤120 ml/kg/min (21). Neonates with low BP (mean BP <25 mmHg), low SVC flow, or low RVO were considered to have abnormal cardiovascular hemodynamics.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 19.0 (SPSS, Chicago, IL). Pearson’s correlation coefficient was used to measure the strength of the linear relationships between various hemodynamic and aEEG parameters. Univariate regression analysis was used to determine additional perinatal and clinical factors significantly related to aEEG parameters. Multiple regression analysis was used to control for the effect of GA and other significant clinical factors (P < 0.05 on univariate analysis) on the relationship between hemodynamic and aEEG parameters. The Mann–Whitney U-test for ANOVA between independent nonparametric samples was used to compare aEEG and EEG values for infants with normal or low cardiovascular parameters. The Friedman two-way ANOVA by ranks for related nonparametric samples was used to assess changes in aEEG and EEG measures over time. A P value of <0.05 was considered to be significant.
Statement of Financial Support
There was no financial support or grant for this research, and the researchers had no financial ties to products in the study.
Disclosure
The authors declared no conflict of interest.
References
Selton D, André M . Prognosis of hypoxic-ischaemic encephalopathy in full-term newborns–value of neonatal electroencephalography. Neuropediatrics 1997;28:276–80.
Toet MC, Hellström-Westas L, Groenendaal F, Eken P, de Vries LS . Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 1999;81:F19–23.
Bowen JR, Paradisis M, Shah D . Decreased aEEG continuity and baseline variability in the first 48 hours of life associated with poor short-term outcome in neonates born before 29 weeks gestation. Pediatr Res 2010;67:538–44.
Hellström-Westas L, Bell AH, Skov L, Greisen G, Svenningsen NW . Cerebroelectrical depression following surfactant treatment in preterm neonates. Pediatrics 1992;89(4 Pt 1):643–7.
Kusaka T, Okubo K, Nagano K, Isobe K, Itoh S . Cerebral distribution of cardiac output in newborn infants. Arch Dis Child Fetal Neonatal Ed 2005;90:F77–8.
Lou HC, Lassen NA, Friis-Hansen B . Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr 1979;94:118–21.
Lundstrøm K, Pryds O, Greisen G . The haemodynamic effects of dopamine and volume expansion in sick preterm infants. Early Hum Dev 2000;57:157–63.
Wardle SP, Yoxall CW, Weindling AM . Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates. J Cereb Blood Flow Metab 2000;20:272–9.
Tyszczuk L, Meek J, Elwell C, Wyatt JS . Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 1998;102(2 Pt 1):337–41.
Greisen G, Pryds O, Rosén I, Lou H . Poor reversibility of EEG abnormality in hypotensive, preterm neonates. Acta Paediatr Scand 1988;77:785–90.
Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM . The relationship between cardiac output, cerebral electrical activity, cerebral fractional oxygen extraction and peripheral blood flow in premature newborn infants. Pediatr Res 2006;60:456–60.
West CR, Groves AM, Williams CE, et al. Early low cardiac output is associated with compromised electroencephalographic activity in very preterm infants. Pediatr Res 2006;59(4 Pt 1):610–5.
Kluckow M, Evans N . Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 2000;82:F182–7.
Evans N, Kluckow M . Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 1996;74:F88–94.
Kluckow M, Evans N . Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 2000;82:F188–94.
Hunt RW, Evans N, Rieger I, Kluckow M . Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 2004;145:588–92.
Hellström-Westas L . Continuous electroencephalography monitoring of the preterm infant. Clin Perinatol 2006;33:633–47, vi.
Osborn DA, Evans N, Kluckow M . Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003;112(1 Pt 1):33–9.
Messick JM Jr, Casement B, Sharbrough FW, Milde LN, Michenfelder JD, Sundt TM Jr . Correlation of regional cerebral blood flow (rCBF) with EEG changes during isoflurane anesthesia for carotid endarterectomy: critical rCBF. Anesthesiology 1987;66:344–9.
Altman DI, Powers WJ, Perlman JM, Herscovitch P, Volpe SL, Volpe JJ . Cerebral blood flow requirement for brain viability in newborn infants is lower than in adults. Ann Neurol 1988;24:218–26.
Jayasinghe D, Gill AB, Levene MI . CBF reactivity in hypotensive and normotensive preterm infants. Pediatr Res 2003;54:848–53.
Kurtis PS, Rosenkrantz TS, Zalneraitis EL . Cerebral blood flow and EEG changes in preterm infants with patent ductus arteriosus. Pediatr Neurol 1995;12:114–9.
Inder TE, Buckland L, Williams CE, et al. Lowered electroencephalographic spectral edge frequency predicts the presence of cerebral white matter injury in premature infants. Pediatrics 2003;111:27–33.
West CR, Harding JE, Williams CE, Gunning MI, Battin MR . Quantitative electroencephalographic patterns in normal preterm infants over the first week after birth. Early Hum Dev 2006;82:43–51.
Maynard DE . EEG processing by the Cerebral Function Monitor (CFM). Ann Anesthesiol Fr 1979;20:170–4.
Clark SJ, Yoxall CW, Subhedar NV . Right ventricular volume measurements in ventilated preterm neonates. Pediatr Cardiol 2004;25:149–53.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, D., Paradisis, M. & Bowen, J. Relationship between systemic blood flow, blood pressure, inotropes, and aEEG in the first 48 h of life in extremely preterm infants. Pediatr Res 74, 314–320 (2013). https://doi.org/10.1038/pr.2013.104
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.104
This article is cited by
-
A practical approach toward interpretation of amplitude integrated electroencephalography in preterm infants
European Journal of Pediatrics (2022)