Abstract
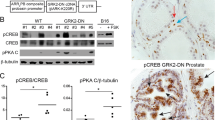
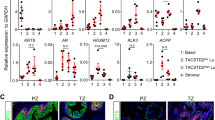
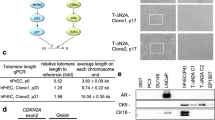
The oncogenic PIM1 kinase has been implicated as a cofactor for c-MYC in prostate carcinogenesis. In this study, we show that in human prostate tumors, coexpression of c-MYC and PIM1 is associated with higher Gleason grades. Using a tissue recombination model coupled with lentiviral-mediated gene transfer we find that Pim1 is weakly oncogenic in naive adult mouse prostatic epithelium. However, it cooperates dramatically with c-MYC to induce prostate cancer within 6-weeks. Importantly, c-MYC/Pim1 synergy is critically dependent on Pim1 kinase activity. c-MYC/Pim1 tumors showed increased levels of the active serine-62 (S62) phosphorylated form of c-MYC. Grafts expressing a phosphomimetic c-MYCS62D mutant had higher rates of proliferation than grafts expressing wild type c-MYC but did not form tumors like c-MYC/Pim1 grafts, indicating that Pim1 cooperativity with c-MYC in vivo involves additional mechanisms other than enhancement of c-MYC activity by S62 phosphorylation. c-MYC/Pim1-induced prostate carcinomas show evidence of neuroendocrine (NE) differentiation. Additional studies, including the identification of tumor cells coexpressing androgen receptor and NE cell markers synaptophysin and Ascl1 suggested that NE tumors arose from adenocarcinoma cells through transdifferentiation. These results directly show functional cooperativity between c-MYC and PIM1 in prostate tumorigenesis in vivo and support efforts for targeting PIM1 in prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Abdulkadir SA, Carbone JM, Naughton CK, Humphrey PA, Catalona WJ, Milbrandt J . (2001a). Frequent and early loss of the EGR1 corepressor NAB2 in human prostate carcinoma. Hum Pathol 32: 935–939.
Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA et al. (2002). Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 22: 1495–1503.
Abdulkadir SA, Qu Z, Garabedian E, Song SK, Peters TJ, Svaren J et al. (2001b). Impaired prostate tumorigenesis in Egr1-deficient mice. Nat Med 7: 101–107.
Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ . (2004). Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett 571: 43–49.
Bachmann M, Hennemann H, Xing PX, Hoffmann I, Moroy T . (2004). The oncogenic serine/threonine kinase Pim-1 phosphorylates and inhibits the activity of Cdc25C-associated kinase 1 (C-TAK1): a novel role for Pim-1 at the G2/M cell cycle checkpoint. J Biol Chem 279: 48319–48328.
Bachmann M, Kosan C, Xing PX, Montenarh M, Hoffmann I, Moroy T . (2006). The oncogenic serine/threonine kinase Pim-1 directly phosphorylates and activates the G2/M specific phosphatase Cdc25C. Int J Biochem Cell Biol 38: 430–443.
Bachmann M, Moroy T . (2005). The serine/threonine kinase Pim-1. Int J Biochem Cell Biol 37: 726–730.
Bhattacharya N, Wang Z, Davitt C, McKenzie IF, Xing PX, Magnuson NS . (2002). Pim-1 associates with protein complexes necessary for mitosis. Chromosoma 111: 80–95.
Chen WW, Chan DC, Donald C, Lilly MB, Kraft AS . (2005). Pim family kinases enhance tumor growth of prostate cancer cells. Mol Cancer Res 3: 443–451.
Cindolo L, Cantile M, Vacherot F, Terry S, de la Taille A . (2007). Neuroendocrine differentiation in prostate cancer: from lab to bedside. Urol Int 79: 287–296.
Cunha GR, Lung B . (1978). The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 205: 181–193.
Deeble PD, Murphy DJ, Parsons SJ, Cox ME . (2001). Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol 21: 8471–8482.
Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K et al. (2001). Delineation of prognostic biomarkers in prostate cancer. Nature 412: 822–826.
di Sant′Agnese PA, de Mesy Jensen KL . (1987). Neuroendocrine differentiation in prostatic carcinoma. Hum Pathol 18: 849–856.
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R et al. (2003). Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4: 223–238.
Garabedian EM, Humphrey PA, Gordon JI . (1998). A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci USA 95: 15382–15387.
Hansel DE, Nakayama M, Luo J, Abukhdeir AM, Park BH, Bieberich CJ et al. (2009). Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate 69: 603–609.
Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R et al. (1998). Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 63: 131–140.
Hu Y, Wang T, Stormo GD, Gordon JI . (2004). RNA interference of achaete-scute homolog 1 in mouse prostate neuroendocrine cells reveals its gene targets and DNA binding sites. Proc Natl Acad Sci USA 101: 5559–5564.
Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ et al. (2003). Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate 55: 219–237.
Kim J, Adam RM, Freeman MR . (2002). Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3 phosphorylation. Cancer Res 62: 1549–1554.
Kim J, Eltoum IE, Roh M, Wang J, Abdulkadir SA . (2009). Interactions between cells with distinct mutations in c-MYC and Pten in prostate cancer. PLoS Genet 5: e1000542.
Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ . (2006). Expression and role of Foxa proteins in prostate cancer. Prostate 66: 1013–1028.
Mochizuki T, Kitanaka C, Noguchi K, Muramatsu T, Asai A, Kuchino Y . (1999). Physical and functional interactions between Pim-1 kinase and Cdc25A phosphatase. Implications for the Pim-1-mediated activation of the c-Myc signaling pathway. J Biol Chem 274: 18659–18666.
Morishita D, Katayama R, Sekimizu K, Tsuruo T, Fujita N . (2008). Pim kinases promote cell cycle progression by phosphorylating and down-regulating p27Kip1 at the transcriptional and posttranscriptional levels. Cancer Res 68: 5076–5085.
Pinski J, Wang Q, Quek ML, Cole A, Cooc J, Danenberg K et al. (2006). Genistein-induced neuroendocrine differentiation of prostate cancer cells. Prostate 66: 1136–1143.
Roh M, Franco OE, Hayward SW, van der Meer R, Abdulkadir SA . (2008). A role for polyploidy in the tumorigenicity of Pim-1-expressing human prostate and mammary epithelial cells. PLoS ONE 3: e2572.
Roh M, Gary B, Song C, Said-Al-Naief N, Tousson A, Kraft A et al. (2003). Overexpression of the oncogenic kinase Pim-1 leads to genomic instability. Cancer Res 63: 8079–8084.
Roh M, Song C, Kim J, Abdulkadir SA . (2005). Chromosomal instability induced by Pim-1 is passage-dependent and associated with dysregulation of cyclin B1. J Biol Chem 280: 40568–40577.
Saris CJ, Domen J, Berns A . (1991). The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J 10: 655–664.
Sauer CG, Roemer A, Grobholz R . (2006). Genetic analysis of neuroendocrine tumor cells in prostatic carcinoma. Prostate 66: 227–234.
Schmidt T, Karsunky H, Rodel B, Zevnik B, Elsasser HP, Moroy T . (1998). Evidence implicating Gfi-1 and Pim-1 in pre-T-cell differentiation steps associated with beta-selection. EMBO J 17: 5349–5359.
Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA et al. (2004). Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res 64: 2270–2305.
Valdman A, Fang X, Pang ST, Ekman P, Egevad L . (2004). Pim-1 expression in prostatic intraepithelial neoplasia and human prostate cancer. Prostate 60: 367–371.
van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T et al. (1989). Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell 56: 673–682.
Vias M, Massie CE, East P, Scott H, Warren A, Zhou Z et al. (2008). Pro-neural transcription factors as cancer markers. BMC Med Genomics 1: 17.
Wafa LA, Palmer J, Fazli L, Hurtado-Coll A, Bell RH, Nelson CC et al. (2007). Comprehensive expression analysis of L-dopa decarboxylase and established neuroendocrine markers in neoadjuvant hormone-treated versus varying Gleason grade prostate tumors. Hum Pathol 38: 161–170.
Wang Z, Bhattacharya N, Mixter PF, Wei W, Sedivy J, Magnuson NS . (2002). Phosphorylation of the cell cycle inhibitor p21Cip1/WAF1 by Pim-1 kinase. Biochim Biophys Acta 1593: 45–55.
Xin L, Ide H, Kim Y, Dubey P, Witte ON . (2003). in vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci USA 100 (Suppl 1): 11896–11903.
Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G et al. (2004). A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol 6: 308–318.
Yuan TC, Veeramani S, Lin MF . (2007). Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 14: 531–547.
Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF . (2003). Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene 22: 6704–6716.
Zhang Y, Wang Z, Li X, Magnuson NS . (2008). Pim kinase-dependent inhibition of c-Myc degradation. Oncogene 27: 4809–4819.
Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P et al. (2006). Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res 66: 7889–7898.
Zippo A, De Robertis A, Serafini R, Oliviero S . (2007). PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nat Cell Biol 9: 932–944.
Acknowledgements
We thank Drs Robert Matusik, Vito Quaranta, Fritz Parl and Susan Kasper for helpful discussions. This work was supported by NIH Grant CA123484 (SAA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Wang, J., Kim, J., Roh, M. et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene 29, 2477–2487 (2010). https://doi.org/10.1038/onc.2010.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.10
Keywords
This article is cited by
-
Characterizing prostate cancer risk through multi-ancestry genome-wide discovery of 187 novel risk variants
Nature Genetics (2023)
-
Longitudinal single-cell transcriptomics reveals distinct patterns of recurrence in acute myeloid leukemia
Molecular Cancer (2022)
-
Exploring the mechanism and experimental verification of puerarin in the treatment of endometrial carcinoma based on network pharmacology and bioinformatics analysis
BMC Complementary Medicine and Therapies (2022)
-
Prostate cancer addiction to oxidative stress defines sensitivity to anti-tumor neutrophils
Clinical & Experimental Metastasis (2022)
-
Identification of PIM1 substrates reveals a role for NDRG1 phosphorylation in prostate cancer cellular migration and invasion
Communications Biology (2021)