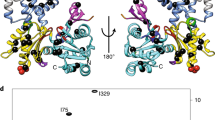
Abstract
Myosins are molecular motor proteins that harness the chemical energy stored in ATP to produce directed force along actin filaments. Complex communication pathways link the catalytic nucleotide-binding region, the structures responsible for force amplification and the actin-binding domain of myosin. We have crystallized the nucleotide-free motor domain of myosin II in a new conformation in which switch I and switch II, conserved loop structures involved in nucleotide binding, have moved away from the nucleotide-binding pocket. These movements are linked to rearrangements of the actin-binding region, which illuminate a previously unobserved communication pathway between the nucleotide-binding pocket and the actin-binding region, explain the reciprocal relationship between actin and nucleotide affinity and suggest a new mechanism for product release in myosin family motors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rayment, I. et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50–58 (1993).
Fisher, A.J. et al. X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP.BeFx and MgADP.AlF4−. Biochemistry 34, 8960–8972 (1995).
Smith, C.A. & Rayment, I. X-ray structure of the magnesium(II).ADP.vanadate complex of the Dictyostelium discoideum myosin motor domain to 1.9 Å resolution. Biochemistry 35, 5404–5417 (1996).
Dominguez, R., Freyzon, Y., Trybus, K.M. & Cohen, C. Crystal structure of a vertebrate smooth muscle myosin motor domain and its complex with the essential light chain: visualization of the pre-power stroke state. Cell 94, 559–571 (1998).
Houdusse, A., Kalabokis, V.N., Himmel, D., Szent-Gyorgyi, A.G. & Cohen, C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell 97, 459–470 (1999).
Kliche, W., Fujita-Becker, S., Kollmar, M., Manstein, D.J. & Kull, F.J. Structure of a genetically engineered molecular motor. EMBO J. 20, 40–46 (2001).
Kollmar, M., Dürrwang, U., Kliche, W., Manstein, D.J. & Kull, F.J. Crystal structure of the motor domain of a class-I myosin. EMBO J. 21, 2517–2525 (2002).
Furch, M., Fujita-Becker, S., Geeves, M.A., Holmes, K.C. & Manstein, D.J. Role of the salt-bridge between switch-1 and switch-2 of Dictyostelium myosin. J. Mol. Biol. 290, 797–809 (1999).
Yount, R.G., Lawson, D. & Rayment, I. Is myosin a 'back door' enzyme? Biophys. J. 68, 47S–49S (1995).
Himmel, D.M. et al. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc. Natl. Acad. Sci. USA 99, 12645–12650 (2002).
Pasqualato, S., Renault, L. & Cherfils, J. Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for 'front-back' communication. EMBO Rep. 3, 1035–1041 (2002).
Smith, C.A. & Rayment, I. Active site comparisons highlight structural similarities between myosin and other P-loop proteins. Biophys. J. 70, 1590–1602 (1996).
Vale, R.D. Switches, latches, and amplifiers: common themes of G proteins and molecular motors. J. Cell Biol. 135, 291–302 (1996).
Kull, F.J., Vale, R.D. & Fletterick, R.J. The case for a common ancestor: kinesin and myosin motor proteins and G proteins. J. Muscle Res. Cell Motil. 19, 877–886 (1998).
Goody, R.S. & Hofmann-Goody, W. Exchange factors, effectors, GAPs and motor proteins: common thermodynamic and kinetic principles for different functions. Eur. Biophys. J. 31, 268–274 (2002).
Volkmann, N. et al. Evidence for cleft closure in actomyosin upon ADP release. Nat. Struct. Biol. 7, 1147–1155 (2000).
Volkmann, N. et al. Myosin isoforms show unique conformations in the actin-bound state. Proc. Natl. Acad. Sci. USA 6, 3227–3232 (2003).
Yengo, C.M. De La Cruz, E.M., Chrin, L.R., Gaffney, D.P. 2nd & Berger, C.L. Actin-induced closure of the actin-binding cleft of smooth muscle myosin. J. Biol. Chem. 277, 24114–24119 (2002).
Friedman, A.L., Geeves, M.A., Manstein, D.J. & Spudich, J.A. Kinetic characterization of myosin head fragments with long-lived myosin-ATP states. Biochemistry 37, 9679–9687 (1998).
Kikkawa, M. et al. Switch-based mechanism of kinesin motors. Nature 411, 439–445 (2001).
Niemann, H.H., Knetsch, M.L., Scherer, A., Manstein, D.J. & Kull, F.J. Crystal structure of a dynamin GTPase domain in both nucleotide-free and GDP-bound forms. EMBO J. 20, 5813–5821 (2001).
Manstein, D.J., Schuster, H.P., Morandini, P. & Hunt, D.M. Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene 162, 129–134 (1995).
Manstein, D.J. & Hunt, D.M. Overexpression of myosin motor domains in Dictyostelium: screening of transformants and purification of the affinity tagged protein. J. Mus. Res. Cell Motil. 16, 325–332 (1995).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Cryst. 26, 795–800 (1993).
Brunger, A.T., et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. and Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Esnouf, R.M. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graph. Model 2, 132–134 (1997).
Merritt, E.A. and Bacon, D.J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Kraulis, P.J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Acknowledgements
We thank W. Kabsch for crystallographic advice, S. Zimmermann and A. Scherer for excellent technical assistance, S. Fujita-Becker for in vitro motility analysis and K.C. Holmes for helpful comments, discussions and continuous support. The work was supported by Molecular Motors project grants from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Reubold, T., Eschenburg, S., Becker, A. et al. A structural model for actin-induced nucleotide release in myosin. Nat Struct Mol Biol 10, 826–830 (2003). https://doi.org/10.1038/nsb987
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsb987
This article is cited by
-
Electrostatic interaction of loop 1 and backbone of human cardiac myosin regulates the rate of ATP induced actomyosin dissociation
Journal of Muscle Research and Cell Motility (2022)
-
Energy transport pathway in proteins: Insights from non-equilibrium molecular dynamics with elastic network model
Scientific Reports (2018)
-
Overview of the mechanism of cytoskeletal motors based on structure
Biophysical Reviews (2018)
-
The Conserved Lysine-265 Allosterically Modulates Nucleotide- and Actin-binding Site Coupling in Myosin-2
Scientific Reports (2017)
-
Structure of actomyosin rigour complex at 5.2 Å resolution and insights into the ATPase cycle mechanism
Nature Communications (2017)