Abstract
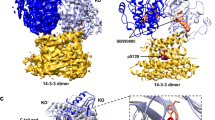
The crystal structure of human racl, a member of the rho family of small G-proteins, complexed with the non-hydrolysable GTP analogue, guanosine-5′-(βγ-imino)triphosphate (GMPPNP), has been determined by X-ray analysis at a resolution of 1.38 Å. Comparison with the structure of H-ras indicates that racl has an extra α-helical domain that is characteristic of the rho G proteins, and may be involved in the signalling pathway of this family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Symons, M. Rho family GTPase: the cytoskeleton and beyond. Trends Biochem. Sci. 21, 178–18 (1996).
Segal, A.W. & Abo, A. Two distinct regions of Ras participate in functional interaction with GDP-GTP exchangers. Trends Biochem. Sci. 18, 43–47 (1993).
de Vos, A.M. et al. Three dimensional structure of human h-ras P21. Science 239, 888–893 (1988).
Pai, E.F. et al. Structure of guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in triphosphate conformation. Nature 341, 209–214 (1989).
Pai, E. et al. Refined crystal structure of triphosphate conformation of H-ras p21 at 1.35 Å resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 9, 2351–2359 (1990).
LOOK : An Integrated Package for Sequence Analysis, Model Building and Function Determination. Molecular Application Group, Palo Alto, Ca. USA. (1990).
Halliday, K.R. Regional homology in GTP-binding proto-oncogene products and elongation of factors. J. Cyclic Nucleo. Prot Phosphory. Rev. 9, 435–448 (1983).
Walker, J.E., Saraste, M., Runswick, M.J. & Gay, N.J. Distantly related sequences in the α- and β-submits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and common nucleotide binding fold. EMBO J. 1, 945–951 (1982).
Feuerstein, J., Goody, R.S. & Webb, M.R. The mechanism of guanosine nucleotide hydrolysis by p21-Ha-ras. J. Biol. Chem. 264, 6188–6190 (1989).
Urzhumtsev, U.G. Density growing: a method for local improvement of electron density. CCP4 Newsletter on Protein Crystallogr. 32, 37–40 (1986).
Freeman, J.L., Abo, A. & Lambeth, J.D. Rac “insert region” is a novel effector region that is implicated in the activation of NADPH oxidase, but not PAK65. J. Biol. Chem. 271, 19794–19801 (1996).
Kwong, C.H., Malech, H.L., Rotrosen, D. & Leto, T.L. Rac “insert region” is a novel effector region that us implicated in the activation of NADPH oxidase, but not PAK65. Biochem. 32, 5711–5717 (1993).
Diekmann, D., Abo, A., Johnston, C., Segal, A.W. & Hall A. Interaction of Rac p67phox and regulation of phagocytic NADPH oxidase activity. Science 265 531–533 (1994).
Joseph, G. & Pick, E. ‘Peptide Walking’ is a novel method for mapping functional domains of proteins. J. Biol. Chem. 270, 29079–29082 (1995).
Leslie, A.G.W. Recent changes to MOSFLM package for processing film and image plate data. CCP4 and ESF-EACMB Newsletter on Protein Crystallography. 26(1992).
CCP4 Collaborative Computational Project, N.4. The CCP4 suit: programs for protein crystallography. Acta Crystallogr. D50 760–763 (1994).
Navaza, AMoRe - An automated package for molecular replacement. J. Acta Crystallogr. A50, 157–163 (1994).
Levitt, M. Accurate modeling of protein conformation by automatic segment matching. J. Mol. Biol. 226, 507–533 (1992).
Lamzin, V.S. & Wilson, K.S. Automated refinement of protein models. Acta Crystallogr. D49, 129–147 (1993).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron-density maps and the location of errors in these models. Acta Crytallogr. A47, 110–119 (1991).
Murshudov, G., Vagin, A. & Dodson, E. in The Refinement of Protein structures Proceedings of Daresbury Study Weekend 37–40 (1996).
Kabsch, W. & Sander, C. Dictionary of protein secondary structure-pattern-recognition and geometrical features. Biopolymers. 22, 2577–2637 (1983).
Kraulis, P.J. MOLSCRIPT - A program to produce both detailed and schematic plots of protein structures. J. Appl. Crytallogr. 24, 946–950 (1991).
Barton, G. ALSCRIPT: a tool to format multiple sequence alignments. J. Prot. Engng. 6, 37–40 (1993).
Brünger, A.T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structure. Nature 355, 472–475 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hirshberg, M., Stockley, R., Dodson, G. et al. The crystal structure of human rac1, a member of the rho-family complexed with a GTP analogue. Nat Struct Mol Biol 4, 147–152 (1997). https://doi.org/10.1038/nsb0297-147
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nsb0297-147
This article is cited by
-
Allosteric inhibition of the guanine nucleotide exchange factor DOCK5 by a small molecule
Scientific Reports (2017)
-
F-box protein complex FBXL19 regulates TGFβ1-induced E-cadherin down-regulation by mediating Rac3 ubiquitination and degradation
Molecular Cancer (2014)
-
A palmitoylation switch mechanism regulates Rac1 function and membrane organization
The EMBO Journal (2012)
-
Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma
Nature Genetics (2012)
-
Crystal structure of Rac1 bound to its effector phospholipase C-β2
Nature Structural & Molecular Biology (2006)