Abstract
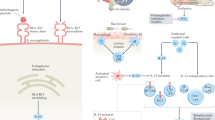
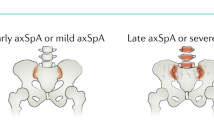
The concept of disease modification in ankylosing spondylitis (AS) incorporates aspects of inflammation, bone destruction and new bone formation. The degree to which inflammation and new bone formation are linked remains conjectural, but data from MRI studies of spinal inflammation support the concept of such coupling; however, these studies also suggest a role for the involvement of noninflammatory pathways, such as those involving bone morphogenetic proteins, wingless proteins and Dickkopf-1, in the formation of new bone. The main clinical outcome that reflects disease modification is the modified Stoke Ankylosing Spondylitis Spine Score, which assesses abnormalities in the anterior vertebral corners of the cervical and lumbar spine. However, radiographic progression can only be reliably detected using this method after at least 2 years, and this delay precludes the conduct of placebo-controlled trials on ethical grounds. Preliminary data using this scoring tool suggest that cyclooxygenase-2-selective NSAIDs might reduce disease progression if used continuously over 2 years. By contrast, three different anti-tumor necrosis factor therapies have shown no impact on radiographic progression. Therapeutic trials recruiting patients early in their disease course and at high risk of radiographic progression constitute a high priority for clinical research in AS.
Key Points
-
Current evidence supports a role for both inflammatory and noninflammatory pathways leading to new bone formation in ankylosing spondylitis (AS)
-
Bone morphogenetic proteins, wingless proteins, Dickkopf-1 (DKK-1) and sclerostin are key mediators regulating new bone formation in AS
-
The interleukin (IL)-23–IL-17 axis is most likely involved in joint inflammation in AS
-
The modified Stoke AS Spine Score used to assess radiographic progression lacks sensitivity to change, and 2 years must elapse before change can be reliably detected
-
Tumor necrosis factor, acting through DKK-1, might be important in repressing new bone formation driven by Wnt proteins in well-established inflammatory lesions
-
Therapeutic trials recruiting patients early in their disease course and at high risk of radiographic progression constitute a high priority for clinical research in AS
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Braun, J. et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum. 48, 1126–1136 (2003).
Maksymowych, W. P. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 53, 703–709 (2005).
Maksymowych, W. P. et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum. 53, 502–509 (2005).
Maksymowych, W. P., Pedersen, S. J., Chiowchanwisawakit, P., Lambert, R. G. W. & Østergaard, M. Systematic MRI evaluation of the frequency and reliability of detection of erosions in the spine of patients with ankylosing spondylitis by the Canada–Denmark MRI working group. Arthritis Rheum. 58, S904 (2008).
McGonagle, D. et al. Distinct topography of erosion and new bone formation in achilles tendon enthesitis: Implications for understanding the link between inflammation and bone formation in spondylarthritis. Arthritis Rheum. 58, 2694–2699 (2008).
Laloux, L. et al. Immunohistological study of entheses in spondyloarthropathies: comparison in rheumatoid arthritis and osteoarthritis. Ann. Rheum. Dis. 60, 316–321 (2001).
Luyten, F. P., Lories, R. J., Verschueren, P., de Vlam, K. & Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 20, 829–848 (2006).
Lories, R. J., Luyten, F. P. & de Vlam, K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res. Therapy 11, 221 (2009).
Lories, R. J. & Luyten, F. P. Bone morphogenetic proteins in destructive and remodeling arthritis. Arthritis Res. Therapy 9, 207 (2007).
Luyten, F. P., Lories, R. J., Verschueren, P., de Vlam, K. & Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 20, 829–848 (2006).
Lories, R. J., Derese, I. & Luyten, F. P. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J. Clin. Invest. 115, 1571–1579 (2005).
Lories, R.J., Derese, I., de Bari, C. & Luyten, F. P. Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum. 56, 489–497 (2007).
Park, M., Chung, S., Park, Y. & Lee, S. Suppression of bone morphogenetic proteins attenuates syndesmophytosis by down-regulating smad pathway in aggrecan-induced spondylitis mice [abstract]. Arthritis Rheum. 58 (Suppl. 2), 484 (2008).
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Schett, G. et al. Blockade of Dickkopf-1 induces fusion of the sacroiliac joints. Ann. Rheum. Dis. doi:10.1136/ard.2008.102046v2.
Wang, N., Morrow, S., Mallon, C. & Maksymowych, W. P. DKK-1 levels are comparably increased in patients with AS and RA, show similar decreases with anti-TNF therapy, and are not associated with markers of bone remodeling [abstract]. Ann. Rheum. Dis. 67 (Suppl. 2), 129 (2008).
Semënov, M., Tamai, K. & He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signalling inhibitor. J. Biol. Chem. 22, 26770–26775 (2005).
Appel, H. et al. Correlation of radiographic progression with serum levels of sclerostin in patients with ankylosing spondylitis [abstract]. Ann. Rheum. Dis. 67 (Suppl. 2), 374 (2008).
Baraliakos, X., Listing, J., Rudwaleit, M., Seiper, J. & Braun, J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res. Ther. 10, R104 (2008).
Maksymowych, W. P. et al. Inflammatory lesions of the spine on MRI predict the development of new syndesmophytes in ankylosing spondylitis: evidence for coupling between inflammation and ankylosis. Arthritis Rheum. 60, 93–102 (2009).
van der Heijde, D. et al. MRI-inflammation of the vertebral unit (vu) only marginally contributes to new syndesmophyte formation in that unit: a multi-level analysis [abstract]. Ann. Rheum. Dis. 67 (Suppl. 2), 130 (2008).
Appel, H. et al. Correlation of histopathological findings and magnetic resonance imaging in the spine of patients with ankylosing spondylitis. Arthritis Res. Ther. 8, R143 (2006).
Lories, R. J., Derese, I. & Luyten, F. P. Inhibition of osteoclasts does not prevent joint ankylosis in a mouse model of spondyloarthritis. Rheumatology 47, 605–608 (2008).
Neidhart, M. et al. Expression of cathepsin K and MMP-1 indicate persistent osteodestructive activity in longstanding ankylosing spondylitis. Ann. Rheum. Dis. 68, 1334–1339 (2009).
Rudwaleit, M. et al. Adalimumab is effective and well tolerated in treating patients with ankylosing spondylitis who have advanced spinal fusion. Rheumatology (Oxford) 48, 551–557 (2009).
Baraliakos, X. et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann. Rheum. Dis. 66, 910–915 (2007).
Wanders, A. et al. Association between radiographic damage of the spine and spinal mobility for individual patients with ankylosing spondylitis: can assessment of spinal mobility be a proxy for radiographic evaluation? Ann. Rheum. Dis. 64, 988–994 (2005).
van der Heijde, D. et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis. Results of a randomized placebo-controlled trial (ASSERT). Arthritis Rheum. 52, 582–591 (2005).
van der Heijde, D. et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 54, 2136−2146 (2006).
Creemers, M. C. et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann. Rheum. Dis. 64, 127–129 (2005).
Wanders, A. J. et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum. 50, 2622–2632 (2004).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
van der Heijde, D. et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 58, 1324–1331 (2008).
van der Heide, D. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 58, 3063–3070 (2008).
van der Heijde, D. M. et al. Adalimumab therapy for ankylosing spondylitis over 2 years does not demonstrate significant inhibition of radiographic progression compared with a historical control group [abstract]. Arthritis Rheum. 58, S413 (2008).
Powell, A., Keeling, S. O., Lambert, R. G. S., Russell, A. S. & Maksymowych, W. P. Scoring of radiographic progression over 2 years with the mSASSS in ankylosing spondylitis: does training improve reliability? [abstract] Arthritis Rheum. 56, S256 (2007).
Baraliakos, X., Listing, J., Rudwaleit, M., Sieper, J. & Braun, J. Development of a radiographic scoring tool for ankylosing spondylitis only based on bone formation: Addition of the thoracic spine improves sensitivity to change. Arthritis Rheum. 61, 764–771 (2009).
Braun, J. et al. Analysing chronic spinal changes in ankylosing spondylitis: a systematic comparison of conventional X rays with magnetic resonance imaging using established and new scoring systems. Ann. Rheum. Dis. 63, 1046–1055 (2004).
Garrett, S. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 21, 2286−2291 (1994).
Zhang, X. et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Invest. 109, 1405–1415 (2002).
van der Heijde, D. M. et al. Prediction of progression of radiographic damage over 4-years in patients with ankylosing spondylitis [abstract]. Ann. Rheum. Dis. 63, OP132 (2004).
Davis, J. C. Jr et al. Recombinant human tumor necrosis factor receptor (Etanercept) for treating ankylosing spondylitis. Arthritis Rheum. 48, 3230–3236 (2003).
Maksymowych, W. P. et al. Etanercept exerts beneficial effects on articular cartilage biomarkers of degradation and turnover in patients with ankylosing spondylitis. J. Rheumatol. 32, 1911–1917 (2005).
Maksymowych, W. P. et al. Beneficial effects of adalimumab on biomarkers reflecting structural damage in patients with ankylosing spondylitis. J. Rheumatol. 35, 2030–2037 (2008).
Baeten, D. et al. Immunomodulatory effects of anti-tumor necrosis factor α therapy on synovium in spondyloarthropathy: Histologic findings in eight patients from an open-label pilot study. Arthritis Rheum. 44, 186–195 (2001).
Baraliakos, X., Davis, J., Tsuji, W. & Braun, J. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis before and after therapy with the tumor necrosis factor alpha receptor fusion protein etanercept. Arthritis Rheum. 52, 1216–1223 (2005).
Lambert, R. G. W. et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis. Arthritis Rheum. 56, 4005–4014 (2007).
Maksymowych, W. P. et al. Serum matrix metalloproteinase 3 is an independent predictor of structural damage progression in patients with ankylosing spondylitis. Arthritis Rheum. 56, 1846–1853 (2007).
Baraliakos, X., Listing, J., von der Recke, A. & Braun, J. The natural course of radiographic progression in ankylosing spondylitis—evidence for major individual variations in a large proportion of patients. J. Rheumatol. 36, 997–1002 (2009).
Romagnani, S. Human Th17 cells. Arthritis Res. Therapy 10, 206–215 (2008).
Jandus, C. et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 58, 2307–2317 (2008).
Bettelli, E., Oukka, M. & Kuchroo, V. K. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8, 345–350 (2007).
Chabaud, M. et al. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12, 1092–1099 (2000).
Singh, R., Aggarwal, A. & Misra, R. Th1/Th17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J. Rheumatol. 34, 2285–2290 (2007).
Cargill, M. et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am. J. Hum. Genet. 80, 273–290 (2007).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Wellcome Trust Case Control Consortium; Australo-Anglo-American Spondylitis Consortium (TASC). Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Rahman, P. et al. Association of interleukin-23 receptor variants with ankylosing spondylitis. Arthritis Rheum. 58, 1020–1025 (2008).
Gottlieb, A. et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet 373, 633–640 (2009).
Costa-Rodriguez, E. V., Napolitani, G., Lanzavecchia, A. & Sallusto, F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949 (2007).
Chiowchanwisawakit, P., Pedersen, S. J., Lambert, R. G. W., Østergaard, M. & Maksymowych, W. P. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation: confirmation of the TNF brake hypothesis [abstract]. Ann. Rheum. Dis. 68 (Suppl. 3), 126 (2009).
Acknowledgements
W. P. Maksymowych is a Scientist of the Alberta Heritage Foundation for Medical Research.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Walter P. Maksymowych declares that he has acted as a consultant for, received speakers bureau (honoraria) from, and received grant or research support from, Amgen/Wyeth, Abbott and Schering-Plough.
Rights and permissions
About this article
Cite this article
Maksymowych, W. Disease modification in ankylosing spondylitis. Nat Rev Rheumatol 6, 75–81 (2010). https://doi.org/10.1038/nrrheum.2009.258
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.258
This article is cited by
-
A novel Anti-ROS osteoblast-specific delivery system for ankylosing spondylitis treatment via suppression of both inflammation and pathological new bone formation
Journal of Nanobiotechnology (2023)
-
Chondrogenesis mediates progression of ankylosing spondylitis through heterotopic ossification
Bone Research (2021)
-
Copy number variation of IL17RA gene and its association with the ankylosing spondylitis risk in Iranian patients: a case-control study
BMC Medical Genetics (2020)
-
Disease Modification in Axial Spondyloarthritis
Current Treatment Options in Rheumatology (2018)
-
Baseline increased 18F-fluoride uptake lesions at vertebral corners on positron emission tomography predict new syndesmophyte development in ankylosing spondylitis: a 2-year longitudinal study
Rheumatology International (2017)