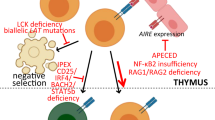
Key Points
-
The study of the genetics and the pathophysiology of immunodeficiencies has a long history of improving our understanding of basic immunological pathways.
-
Recent advances in laboratory diagnostics and sequencing have led to substantial advances in new aetiologies and pathways of immune diseases; some of these diseases have previously been described but others are newly identified.
-
Hypomorphic mutations in immune-related genes in humans result in disease phenotypes that are not predicted by null mutations in mice. Such findings highlight the differences between the mouse and human immune systems. These hypomorphic mutations also enable the study of the function of certain proteins in cell lineages that are normally absent in mice that have null mutations.
-
One example of the results of such investigations can be found in patients who have gene deficiencies that impair their immunity to Epstein–Barr virus. The study of such individuals led to the discovery of a role for magnesium as a second messenger in lymphocytes.
-
The study of a cohort of patients with familial cold urticaria led to the identification of phospholipase Cγ2 mutations, which could render cellular signalling hyporesponsive at body temperature but spontaneously active in the cold.
-
Patients with familial myelodysplasia and acute myeloid leukaemia, as well as patients who show increased susceptibility to a wide-range of infections, carry heterogeneous mutations in the transcription factor GATA-binding protein 2. These individuals provide an opportunity to study this transcription factor in the context of infection and oncogenesis.
Abstract
A recent surge in newly described primary immunodeficiencies (PIDs) has highlighted new physiological and pathophysiological pathways that affect the immune system. Furthermore, the study of individuals with PIDs has substantially improved our understanding of basic cellular and signalling pathways in host defence and immune regulation. Single-gene defects can lead to disease manifestations that range from extremely narrow infectious phenotypes to remarkably broad multisystem effects. Hypomorphic or hypermorphic gene mutations often occur in human diseases; when coupled with the fact that humans are exposed to naturally encountered antigens and pathogens, this helps to make the case that the study of immunological diseases in humans should be at the forefront of basic immunological research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ochs, H. D. & Hitzig, W. H. History of primary immunodeficiency diseases. Curr. Opin. Allergy Clin. Immunol. 12, 577–587 (2012).
Bruton, O. C. Agammaglobulinemia. Pediatrics 9, 722–728 (1952).
Casanova, J. L. & Abel, L. Primary immunodeficiencies: a field in its infancy. Science 317, 617–619 (2007).
Davis, M. M. Immunology taught by humans. Sci. Transl. Med. 4, 117fs112 (2012).
Zhang, Q. et al. Combined immunodeficiency associated with DOCK8 mutations. N. Engl. J. Med. 361, 2046–2055 (2009).
Sanal, O. et al. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J. Clin. Immunol. 32, 698–708 (2012).
Jabara, H. H. et al. DOCK8 functions as an adaptor that links TLR–MyD88 signaling to B cell activation. Nature Immunol. 13, 612–620 (2012).
Picard, C. et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine 89, 403–425 (2010).
Weller, S. et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients. Blood 120, 4992–5001 (2012).
He, B. et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nature Immunol. 11, 836–845 (2010).
Isnardi, I. et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity 29, 746–757 (2008).
Randall, K. L. et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J. Exp. Med. 208, 2305–2320 (2011).
Randall, K. L., Lambe, T., Goodnow, C. C. & Cornall, R. J. The essential role of DOCK8 in humoral immunity. Dis. Markers 29, 141–150 (2010).
Mizesko, M. C. et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 131, 840–848 (2013).
Harada, Y. et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119, 4451–4461 (2012).
Mueller, P. et al. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nature Immunol. 9, 424–431 (2008).
Shiow, L. R. et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nature Immunol. 9, 1307–1315 (2008).
Moshous, D. et al. Whole-exome sequencing identifies coronin-1A deficiency in 3 siblings with immunodeficiency and EBV-associated B-cell lymphoproliferation. J. Allergy Clin. Immunol. 131, 1594–1603.e9 (2013).
Pan, D. The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Nehme, N. T. et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119, 3458–3468 (2012).
Abdollahpour, H. et al. The phenotype of human STK4 deficiency. Blood 119, 3450–3457 (2012).
Crequer, A. et al. Inherited MST1 deficiency underlies susceptibility to EV-HPV infections. PLoS ONE 7, e44010 (2012).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Mou, F. et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J. Exp. Med. 209, 741–759 (2012).
Palendira, U. et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 9, e1001187 (2011).
Palendira, U. et al. Expansion of somatically reverted memory CD8+ T cells in patients with X-linked lymphoproliferative disease caused by selective pressure from Epstein-Barr virus. J. Exp. Med. 209, 913–924 (2012).
Li, F. Y. et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 475, 471–476 (2011). This study is a prime example of the discovery of a novel biological pathway. A previously unrecognized role for magnesium as a second messenger for signalling was identified through the study of a discrete population of immunodeficient patients
Niehues, T., Perez-Becker, R. & Schuetz, C. More than just SCID — the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin. Immunol. 135, 183–192 (2010).
Omenn, G. S. Familial reticuloendotheliosis with eosinophilia. N. Engl. J. Med. 273, 427–432 (1965).
Schuetz, C. et al. An immunodeficiency disease with RAG mutations and granulomas. N. Engl. J. Med. 358, 2030–2038 (2008). This is the first report to show that hypomorphic mutations in RAG genes could lead to phenotypes that are quite distinct from Omenn syndrome or SCID. These patients provide the opportunity to study the roles of RAG proteins in human V(D)J gene recombination, the affect they have on TCR repertoires and a new pathophysiological role for RAG function in granulomatous disease.
De Ravin, S. S. et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood 116, 1263–1271 (2010).
Avila, E. M. et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics 126, e1248–e1252 (2010).
Kuijpers, T. W. et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood 117, 5892–5896 (2011).
de Villartay, J. P. et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J. Clin. Invest. 115, 3291–3299 (2005).
Marrella, V. et al. A hypomorphic R229Q Rag2 mouse mutant recapitulates human Omenn syndrome. J. Clin. Invest. 117, 1260–1269 (2007).
Walter, J. E. et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J. Exp. Med. 207, 1541–1554 (2010).
Cassani, B. et al. Homeostatic expansion of autoreactive immunoglobulin-secreting cells in the Rag2 mouse model of Omenn syndrome. J. Exp. Med. 207, 1525–1540 (2010).
Milner, J. D., Ward, J. M., Keane-Myers, A. & Paul, W. E. Lymphopenic mice reconstituted with limited repertoire T cells develop severe, multiorgan, Th2-associated inflammatory disease. Proc. Natl Acad. Sci. USA 104, 576–581 (2007).
Rucci, F. et al. Abnormalities of thymic stroma may contribute to immune dysregulation in murine models of leaky severe combined immunodeficiency. Front. Immunol. http://dx.doi.org/10.3389/fimmu.2011.00015 (2013).
Conley, M. E. et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J. Exp. Med. 209, 463–470 (2012).
Fruman, D. A. et al. Hypoglycaemia, liver necrosis and perinatal death in mice lacking all isoforms of phosphoinositide 3-kinase p85α. Nature Genet. 26, 379–382 (2000).
Datta, S. & Milner, J. D. Altered T-cell receptor signaling in the pathogenesis of allergic disease. J. Allergy Clin. Immunol. 127, 351–354 (2011).
Gandhi, C., Healy, C., Wanderer, A. A. & Hoffman, H. M. Familial atypical cold urticaria: description of a new hereditary disease. J. Allergy Clin. Immunol. 124, 1245–1250 (2009).
Ombrello, M. J. et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 366, 330–338 (2012).
Wang, D. et al. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13, 25–35 (2000).
Yu, P. et al. Autoimmunity and inflammation due to a gain-of-function mutation in phospholipase Cγ2 that specifically increases external Ca2+ entry. Immunity 22, 451–465 (2005).
Abe, K. et al. A novel N-ethyl-N-nitrosourea-induced mutation in phospholipase Cγ2 causes inflammatory arthritis, metabolic defects, and male infertility in vitro in a murine model. Arthritis Rheum. 63, 1301–1311 (2011).
Freeman, A. F. et al. Coronary artery abnormalities in Hyper-IgE syndrome. J. Clin. Immunol. 31, 338–345 (2011).
Fairchild, K. D., Singh, I. S., Carter, H. C., Hester, L. & Hasday, J. D. Hypothermia enhances phosphorylation of IκB kinase and prolongs nuclear localization of NF-κB in lipopolysaccharide-activated macrophages. Am. J. Physiol. Cell Physiol. 289, C1114–C1121 (2005).
Zhou, Q. et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 91, 713–720 (2012). References 44 and 50 show the remarkable complexity and the diversity of cellular and clinical phenotypes, including allergy, immunodeficiency and autoinflammation, which emerge from two different types of gain-of-function mutations in PLCG2.
Casanova, J. L., Abel, L. & Quintana-Murci, L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu. Rev. Immunol. 29, 447–491 (2011).
Sancho-Shimizu, V. et al. Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency. J. Clin. Invest. 121, 4889–4902 (2011).
Guo, Y. et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exp. Med. 208, 2083–2098 (2011).
Perez de Diego, R. et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity 33, 400–411 (2010).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317, 1522–1527 (2007).
Casrouge, A. et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314, 308–312 (2006).
Herman, M. et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J. Exp. Med. 209, 1567–1582 (2012).
Lafaille, F. G. et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491, 769–773 (2012). Using induced pluripotent stem cells, this study shows that the increased susceptibility to HSV-1 encephalitis that occurs in patients with defects in TLR3 is due to intrinsic defects in innate-sensing mechanisms in central nervous system-resident cells, including neurons and oligodendrocytes.
Vinh, D. C. et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 115, 1519–1529 (2010).
Hsu, A. P. et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 118, 2653–2655 (2011).
Bigley, V. et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J. Exp. Med. 208, 227–234 (2011).
Ostergaard, P. et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nature Genet. 43, 929–931 (2011).
Hahn, C. N. et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nature Genet. 43, 1012–1017 (2011). References 60, 62 and 63 provide examples of multiple discrete phenotypes that arise from mutations in a single gene. These studies show how defects in a single transcription factor can lead to myeloid dysplasia and defective host defence.
Rodrigues, N. P. et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 106, 477–484 (2005).
Kumar, M. S. et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 149, 642–655 (2012).
Vicente, C. et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia 26, 550–554 (2012).
Zhang, S. J. et al. Gain-of-function mutation of GATA-2 in acute myeloid transformation of chronic myeloid leukemia. Proc. Natl Acad. Sci. USA 105, 2076–2081 (2008).
Johnson, K. D. et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J. Clin. Invest. 122, 3692–3704 (2012).
Mace, E. M. et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56bright subset. Blood 121, 2669–2677 (2013).
Biron, C. A., Byron, K. S. & Sullivan, J. L. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320, 1731–1735 (1989).
Kazenwadel, J. et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 119, 1283–1291 (2012).
Cuellar-Rodriguez, J. et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood 118, 3715–3720 (2011).
Marquis, J. F. et al. Interferon regulatory factor 8 regulates pathways for antigen presentation in myeloid cells and during tuberculosis. PLoS Genet. 7, e1002097 (2011).
Holtschke, T. et al. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 (1996).
Hambleton, S. et al. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 365, 127–138 (2011).
Chen, Z. & O'Shea, J. J. Th17 cells: a new fate for differentiating helper T cells. Immunol. Res. 41, 87–102 (2008).
Milner, J. D. et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008).
Ma, C. S. et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 205, 1551–1557 (2008).
de Beaucoudrey, L. et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 205, 1543–1550 (2008).
Renner, E. D. et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced TH17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J. Allergy Clin. Immunol. 122, 181–187 (2008).
Puel, A. et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011).
Kisand, K. et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 207, 299–308 (2010).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010). References 81–83 were the first to highlight the remarkably limited infectious phenotype (that is, increased susceptibility to chronic mucocutaneous candidiasis) that occurs in patients with focal defects in IL-17-mediated signalling. By contrast, IL-17 has been shown to protect against a wider range of infections in mice.
Ferwerda, B. et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 (2009).
Glocker, E. O. et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N. Engl. J. Med. 361, 1727–1735 (2009).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Holland, S. M. et al. STAT3 Mutations in the Hyper-IgE Syndrome. N. Engl. J. Med. 357, 1608–1619 (2007).
Speckmann, C. et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin. Immunol. 129, 448–454 (2008).
Avery, D. T. et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207, 155–171 (2010).
Schmitt, N. et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity 31, 158–169 (2009).
Ma, C. S. et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood 119, 3997–4008 (2012).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Siegel, A. M. et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011).
Kida, H. et al. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am. J. Pathol. 172, 1542–1554 (2008).
Kovacic, J. C. et al. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J. Clin. Invest. 120, 303–314 (2010).
Chandesris, M. O. et al. Frequent and widespread vascular abnormalities in human signal transducer and activator of transcription 3 deficiency. Circ. Cardiovasc. Genet. 5, 25–34 (2012).
Nieminen, P. et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am. J. Hum. Genet. 89, 67–81 (2011).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009).
Gough, D. J. et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324, 1713–1716 (2009).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Liu, L. et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J. Exp. Med. 208, 1635–1648 (2011).
van de Veerdonk, F. L. et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N. Engl. J. Med. 365, 54–61 (2011).
Boisson-Dupuis, S. et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr. Opin. Immunol. 24, 364–378 (2012).
Smeekens, S. P. et al. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS ONE 6, e29248 (2011).
Sampaio, E. P. et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J. Allergy Clin. Immunol. 131, 1624–1634 (2013).
Uzel, G. et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J. Allergy Clin. Immunol. 131, 1611–1623.e3 (2013).
Romberg, N. et al. Gain-of-function STAT1 mutations are associated with PD-L1 overexpression and a defect in B-cell survival. J. Allergy Clin. Immunol. 131, 1691–1693 (2013).
Yang, X. P. et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nature Immunol. 12, 247–254 (2011).
Yoshimura, A., Nishinakamura, H., Matsumura, Y. & Hanada, T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res. Ther. 7, 100–110 (2005).
Grimbacher, B. et al. Hyper-IgE syndrome with recurrent infections — an autosomal dominant multisystem disorder. N. Engl. J. Med. 340, 692–702 (1999).
Rezaei, N., Notarangelo, L. & Aghamohammadi, A. Primary Immunodeficiency Diseases: Definition, Diagnosis, and Management 59–64 (Springer, 2008).
Lean, I. S., McDonald, V. & Pollok, R. C. The role of cytokines in the pathogenesis of Cryptosporidium infection. Curr. Opin. Infect. Dis. 15, 229–234 (2002).
Kotlarz, D. et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J. Exp. Med. 210, 433–443 (2013).
Recher, M. et al. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood 118, 6824–6835 (2011).
de Totero, D. et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood 107, 3708–3715 (2006).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Glocker, E. O. et al. Infant colitis — it's in the genes. Lancet 376, 1272 (2010).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033–2045 (2009).
Engelhardt, K. R. et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J. Allergy Clin. Immunol. 131, 825–830 (2012). This study shows that colitis can be a monogenic disease and can be cured by bone marrow transplantation. It highlights the important role of IL-10 signalling in haematopoietic cells in preventing intestinal inflammation.
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Sayos, J. et al.The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature 395, 462–469 (1998).
Chaigne-Delalande, B. et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 341, 186–191 (2013).
Acknowledgements
This work has been supported by the Division of Intramural Research, US National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- T cell receptor excision circles
-
(TRECs). DNA episomes that are normally produced during the thymic maturation of T cells, specifically during recombination of the T cell receptor genes.
- Wiskott–Aldrich syndrome protein
-
(WASP). An actin regulator that is involved in the formation of the immunological synapse. Mutations in WASP cause a life-threatening X-linked immunodeficiency that is characterized by thrombocytopaenia with small platelets, eczema, recurrent infections and an increased incidence of autoimmune manifestations and malignancies.
- Recombinase activating gene 1
-
(RAG1). RAG1 and RAG2 encode the RAG proteins, which are involved in creating the double-stranded DNA breaks that are required to produce the rearranged gene segments encoding the complete protein chains of B cell and T cell receptors.
- Mycobacterium avium complex
-
A complex of mycobacterial species that are ubiquitous in the environment and that can cause disease in immunocompromised individuals.
- Immunoscope assays
-
Techniques used to assess the diversity of the T cell receptor (TCR) repertoire in a population of T cells. They use a reverse transcription PCR-based approach to assess the length of the complementarity determining region 3 of the variable genes encoding the TCR.
Rights and permissions
About this article
Cite this article
Milner, J., Holland, S. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol 13, 635–648 (2013). https://doi.org/10.1038/nri3493
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3493
This article is cited by
-
Primary immunodeficiency diseases in a tuberculosis endemic region: challenges and opportunities
Genes & Immunity (2019)
-
Ikaros family zinc finger 1 regulates dendritic cell development and function in humans
Nature Communications (2018)
-
Life with a Primary Immune Deficiency: a Systematic Synthesis of the Literature and Proposed Research Agenda
Journal of Clinical Immunology (2016)
-
Predispositions to Lymphoma: A Practical Review for Genetic Counselors
Journal of Genetic Counseling (2016)
-
Coronin-1A: Immune Deficiency in Humans and Mice
Journal of Clinical Immunology (2015)