Abstract
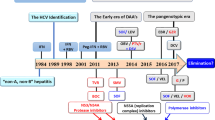
Chronic infection with HCV has an estimated prevalence of 1.6–2.0% worldwide and is a major cause of liver-related death. The first attempts to halt the progression of infection relied on the empirical use of interferon (IFN), a naturally occurring cytokine that is implicated in antiviral innate immunity. The first studies of this treatment in the early 1990s, however, led to disappointing response rates. These response rates subsequently improved with the empirical addition of the guanosine analog ribavirin to the treatment regimen. To improve the effectiveness and tolerability of the three times per week therapeutic schedule of IFN, two forms of pegylated interferon (PEG-IFN) were developed in the early 2000s—PEG-IFN-α2a and PEG-IFN-α2b. These two compounds differ markedly in size, structure, site of attachment of the polyethylene glycol moiety and type of bond involved in pegylation, which ultimately confer different pharmacokinetics and biological activity. Unsurprisingly, researchers question whether the two PEG-IFNs also differ in clinical effectiveness, but the re-analysis of restrospective studies and the results of three head-to-head studies have left this issue open. We have, therefore, scrutinized the design and conduct of all available studies to unravel the reasons behind the therapeutic differences between PEG-IFN-α2a and PEG-IFN-α2b.
Key Points
-
Pegylated interferons (PEG-IFNs) α2a and α2b differ considerably in terms of their pharmacokinetics and biological activity as a direct consequence of their different pegylation characteristics
-
PEG-IFN-α2a or PEG-IFN-α2b, in combination with ribavirin, are both approved for the treatment of chronic hepatitis C
-
Direct comparisons of the two PEG-IFN regimens are difficult because of their different dosing, and the different ribavirin doses and dose-reduction strategies used
-
The PEG-IFN-α2a regimen had higher response rates than the PEG-IFN-α2b regimen in two head-to-head, randomized studies, and a meta-analysis of 13 studies in patients with chronic hepatitis C
-
Further studies are needed to assess whether the outcomes of patients treated with the two therapeutic regimens are differentially influenced by host and viral factors
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Armstrong, G. L. et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144, 705–714 (2006).
Esteban, J. I, Sauleda, S. & Quer, J. The changing epidemiology of hepatitis C virus infection in Europe. J. Hepatol. 48, 148–162 (2008).
Bosetti, C. et al. Worldwide mortality from cirrhosis: an update to 2002. J. Hepatol. 46, 827–839 (2007).
Poynard, T. et al. Impact of pegylated interferon-α2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology 122, 1303–1313 (2002).
Bruno, S. et al. Sustained virological response to interferon-α is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology 45, 579–587 (2007).
Hoofnagle, J. H. et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 315, 1575–1578 (1986).
Houghton, M. The long and winding road leading to the identification of the hepatitis C virus. J. Hepatol. 51, 939–948 (2009).
Poynard, T. et al. Randomised trial of interferon-α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon-α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352, 1426–1432 (1998).
McHutchison, J. G. et al. Interferon-α2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339, 1485–1492 (1998).
Harris, J. M., Martin, N. E. & Modi, M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 40, 539–551 (2001).
Glue, P. et al. Pegylated interferon-α2b: pharmacokinetics, pharmacodynamics, safety and preliminary efficacy data. Clin. Pharmacol. Ther. 68, 556–567 (2000).
Bailon, P. et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon-α2a for the treatment of hepatitis C. Bioconjug. Chem. 12, 195–202 (2001).
Caliceti, P. Pharmacokinetics of pegylated interferons: what is misleading? Dig. Liver Dis. 36 (Suppl. 3), S334–S339 (2004).
Foster, G. R. Pegylated interferons: chemical and clinical differences. Aliment. Pharmacol. Ther. 20, 825–830 (2004).
McHutchison, J. G. et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N. Engl. J. Med. 361, 580–593 (2009).
Rumi, M. G. et al. Randomized study of peginterferon-α2a plus ribavirin vs peginterferon-α2b plus ribavirin in chronic hepatitis C. Gastroenterology 138, 108–115 (2010).
Ascione, A. et al. Peginterferon alfa-2a plus ribavirin is more effective than peginterferon alfa-2b plus ribavirin for treating chronic hepatitis C virus infection. Gastroenterology 138, 116–122 (2010).
Samuel, C. E. Antiviral actions of interferons. Clin. Microbiol. Rev. 14, 778–809 (2010).
Sheppard, P. et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4, 63–68 (2003).
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Thomas, D. L. et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801 (2009).
Katze, M. G., He, Y. & Gale, M. Jr. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 (2002).
Sen, G. C. & Ransohoff, R. M. Interferon-induced antiviral actions and their regulation. Adv. Virus Res. 42, 57–102 (1993).
Pawlotsky, J. M. Current and future concepts in hepatitis C therapy. Semin. Liver Dis. 25, 72–83 (2005).
Pawlotsky, J. M. Therapy of hepatitis C: from empiricism to eradication. Hepatology 43 (Suppl. 1), S207–S220 (2006).
Peters, M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology 23, 909–916 (1996).
Baron, S. et al. The interferons. Mechanisms of action and clinical applications. JAMA 266, 1375–1383 (1991).
Rumi, M. et al. A prospective, randomized trial comparing lymphoblastoid to recombinant interferon alfa 2a as therapy for chronic hepatitis C. Hepatology 24, 1366–1370 (1996).
Pedder, S. C. Pegylation of interferon alfa: structural and pharmacokinetic properties. Semin. Liver Dis. 23 (Suppl. 1), 19–22 (2003).
Aghemo, A., Rumi, M. G. & Colombo, M. Pegylated IFN-α2a and ribavirin in the treatment of hepatitis C. Expert Rev. Anti Infect. Ther. 7, 925–935 (2009).
Silva, M. et al. A randomised trial to compare the pharmacokinetic, pharmacodynamic, and antiviral effects of peginterferon alfa-2b and peginterferon alfa-2a in patients with chronic hepatitis C (COMPARE). J. Hepatol. 45, 204–213 (2006).
Grace, M. et al. Structural and biologic characterization of pegylated recombinant IFN-α2b. J. Interferon Cytokine Res. 21, 1103–1115 (2001).
Formann, E., Jessner, W., Bennett, L. & Ferenci, P. Twice-weekly administration of peginterferon-α-2b improves viral kinetics in patients with chronic hepatitis C genotype 1. J. Viral Hepat. 10, 271–276 (2003).
Lurie, Y. et al. Optimal dosing frequency of pegylated interferon alfa-2b monotherapy for chronic hepatitis C virus infection. Clin. Gastroenterol. Hepatol. 3, 610–615 (2005).
Manns, M. P. et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001).
Fried, M. W. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347, 975–982 (2002).
Garattini, S. & Bertele', V. Ethics in clinical research. J. Hepatol. 51, 792–797 (2009).
Witthoeft, T. et al. Efficacy and safety of peginterferon alfa-2a or alfa-2b plus ribavirin in the routine daily treatment of chronic hepatitis C patients in Germany: the PRACTICE Study. J. Hepatol. 48 (Suppl. 2), S315 (2008).
Almasio, P. L. et al. Efficacy of PEG-IFN alfa-2b vs PEG-IFN alfa-2a plus ribavirin regimens in treatment-naive chronic HCV patients: a cumulative meta-analysis of retrospective data from 6 clinic sites [abstract 1205]. Hepatology 42 (Suppl. 1), 671A (2005).
Manos, M. et al. Determinants of sustained viral response (SVR) in patients receiving PEG-interferon/ribavirin therapy for hepatitis C: results from a diverse managed care population. Presented at the 13th International Symposium on Viral Hepatitis and Liver diseases (2009).
Backus, L. I., Boothroyd, D. B., Phillips, B. R. & Mole, L. A. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology 46, 37–47 (2007).
Thuy, P. T. & Dat, H. T. Comparison between the two Peginterferons alfa in the treatment of chronic hepatitis C [abstract 332]. Hepatology 46 (4 Suppl. 1), 387A–388A (2007).
Hadziyannis, S. J. et al. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140, 346–355 (2004).
Sulkowski, M. et al. Final results of the ideal (individualized dosing efficacy versus flat dosing to assess optimal pegylated interferon therapy) phase IIIB study [abstract 991]. J. Hepatol. 48 (Suppl. 2), S370–S371 (2008).
Ascione, A. et al. Peginterferon alpha-2a plus ribavirin versus Peginterferon alpha-2b plus ribavirin in naive patients with chronic hepatitis C virus infection: results of a prospective randomised trial [abstract 990]. J. Hepatol 48 (Suppl. 2), S370 (2008).
Rumi, M. G. et al. Randomized study comparing peginterferon-alfa2a plus ribavirin and peginterferon-alfa2b plus ribavirin in naive patients with chronic hepatitis C: final results of the Milan safety tolerability (MIST) study [abstract 212]. Hepatology 48 (Suppl.), 404A (2008).
Rumi, M. G. Pegylated interferon α2b versus pegylated interferon α2a for chronic hepatitis C: the unreached goal of superiority. J. Hepatol. 51, 1097–1099 (2009).
Craxì, A. PEG IFN alfa-2a vs. alfa-2b: and the winner is? J. Hepatol. 52, 133–135 (2010).
Schulze Zur Wiesch, J. & Lohse, A. W. Results of the IDEAL trial: “Mirror, mirror on the wall. which's the fairest peg-interferon of them all?”. Hepatology 50, 2034–2037 (2009).
Aghemo, A. & Colombo, M. Peginterferon alfa-2b versus peginterferon alfa-2a with ribavirin for the treatment of chronic hepatitis C: the pursuit of an ideal. Gastroenterology 138, 386–389 (2010).
Zeuzem, S. et al. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J. Hepatol. 43, 250–257 (2005).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-α and ribavirin therapy. Nat. Genet. 41, 1100–1104 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41, 1105–1109 (2009).
Rauch, A. et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138, 1338–1345 (2010).
McCarthy, J. J. et al. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology 138, 2307–2314 (2010).
Jacobson, I. M. et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology 46, 971–981 (2007).
McHutchison, J. G. et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 123, 1061–1069 (2002).
Reddy, K. R. et al. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon alfa-2a/ribavirin treatment. Clin. Gastroenterol. Hepatol. 5, 124–129 (2007).
Croswell, J. M. & Kramer, B. S. Clinical trial design and evidence-based outcomes in the study of liver diseases. J. Hepatol. 50, 817–826 (2009).
Awad, T. et al. PegInterferon-alfa2a is associated with higher sustained virological than PegInterferon-alfa2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 51, 1176–1184 (2010).
Kau, A., Vermehren, J. & Sarrazin, C. Treatment predictors of a sustained virologic response in hepatitis B and C. J. Hepatol. 49, 634–651 (2008).
Bruno, S. et al. Efficacy and safety of peginterferon alfa-2a (40kD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 51, 388–397 (2010).
Aghemo, A. et al. The pattern of pegylated interferon-alpha2b and ribavirin treatment failure in cirrhotic patients depends on hepatitis C virus genotype. Antivir. Ther. 14, 577–584 (2009).
Prati, G. M. et al. The influence of liver fibrosis on the outcome of pegylated interferon and ribavirin anti-HCV therapy: a sub-analysis of the MIST study [abstract 818]. Hepatology 50 (Suppl. 4), 687 (2009).
Di Bisceglie, A. M., Ghalib, R. H., Hamzeh, F. & Rustgi, V. K. Early virologic response after peginterferon alpha-2a plus ribavirin or peginterferon alpha-2b plus ribavirin treatment in patients with chronic hepatitis C. J. Viral Hep 14, 721–729 (2007).
Zeuzem, S. Do differences in pegylation of interferon alfa matter? Gastroenterology 138, 34–36 (2010).
McHutchison, J. G. et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N. Engl. J. Med. 360, 1827–1838 (2009).
Hézode, C. et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N. Engl. J. Med. 360, 1839–1850 (2009).
Kwo, P. et al. HCV-SPRINT 1 final results: SVR 24 from a phase II study of Boceprevir plus PegIntron (PegIFN-alfa2b)/Ribavirin in treatment-naïve subjects with genotype 1 chronic hepatitis C [abstract 4]. J. Hepatol. 50 (Suppl. 1), A4 (2009).
Sarrazin, C. & Zeuzem, S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology 138, 447–462 (2010).
Gane, E. J. et al. First-in-man demonstration of potent antiviral activity with a nucleoside polymerase (R7128) and protease (R7227/ITMN-191) inhibitor combination in hcv: safety, pharmacokinetics, and virologic results from inform-1 [abstract 1046]. J. Hepatol. 50 (Suppl.), S380 (2009).
Study to determine the effectiveness of antiviral combination therapy to treat hepatitis C virus (HCV)-infected patients who have previously failed standard of care ClinicalTrials.gov[online], (2010).
Author information
Authors and Affiliations
Contributions
A. Aghemo and M. G. Rumi contributed equally to researching the data for the article. A. Aghemo, M. G. Rumi and M. Colombo contributed equally to substantial discussions of the content, to writing the article and to reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. G. Rumi declares that she has a speaking and teaching role with Roche.
M. Colombo declares that he has received grant and research support from Schering-Plough, Roche, Novartis and Bristol-Myers Squibb, he is on the advisory committees for Schering-Plough, Roche, Novartis, Vertex, Bristol-Myers Squibb, Gilead Sciences and Bayer and he has speaking and teaching roles with Schering-Plough, Roche, Novartis, Vertex, Gilead Sciences, Bristol-Myers Squibb and Bayer.
A. Aghemo delcares no competing interests.
Rights and permissions
About this article
Cite this article
Aghemo, A., Rumi, M. & Colombo, M. Pegylated interferons α2a and α2b in the treatment of chronic hepatitis C. Nat Rev Gastroenterol Hepatol 7, 485–494 (2010). https://doi.org/10.1038/nrgastro.2010.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2010.101
This article is cited by
-
Direct and indirect effects of IFN-α2b in malignancy treatment: not only an archer but also an arrow
Biomarker Research (2022)
-
Harnessing Type I IFN Immunity Against SARS-CoV-2 with Early Administration of IFN-β
Journal of Clinical Immunology (2021)
-
Efficacy and safety of interferon-α2b spray in the treatment of hand, foot, and mouth disease: a multicenter, randomized, double-blind trial
Archives of Virology (2016)
-
A Retrospective Study to Examine Healthcare Costs Related to Cardiovascular Events in Individuals with Hyperlipidemia
Advances in Therapy (2015)
-
Thyroid disease is a favorable prognostic factor in achieving sustained virologic response in chronic hepatitis C undergoing combination therapy: A nested case control study
BMC Endocrine Disorders (2011)