Key Points
-
A stem-cell niche is a restricted locale in an organ that supports the self-renewing division of stem cells and so prevents them from differentiating.
-
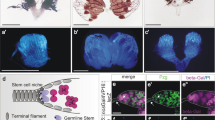
Cap and terminal filament cells and hub cells constitute the stem-cell niche in the fly ovary and testis, respectively.
-
Both ovarian and testicular stem-cell niches are dual-functional niches that emanate signals that are essential for the maintenance of both germline and somatic stem cells.
-
The ovarian niche generates several signalling pathways, including the Decapentaplegic (Dpp) and female sterile (1) Yb (Yb)/Piwi/Hedgehog (Hh)-mediated pathways, which act in parallel to maintain germline stem cells.
-
The Yb/Piwi/Hh-mediated pathway bifurcates downstream of Yb, with the Piwi and Hh branches mainly responsible for the maintenance of germline and somatic stem cells, respectively.
-
Cell adhesions through adherens junctions anchor both germline and somatic stem cells to the niche.
-
The testicular niche generates the Unpaired ligand that is essential for the self-renewal of both germline and somatic stem cells.
-
In contrast to its oogenic function, the Dpp pathway functions to restrict the proliferation of gonialblasts and spermatogonia in males. Epidermal growth factor receptor and Raf also have this function.
-
The organization and signalling pathways of the fly niches provide guiding principles for studying stem-cell niches in mammalian systems.
Abstract
Stem cells are characterized by their ability to self-renew and to produce numerous differentiated cell types, and are directly responsible for generating and maintaining tissues and organs. This property has long been attributed to the instructive signals that stem cells receive from their microenvironment — the so-called 'stem-cell niche'. Studies of stem cells in the Drosophila gonad have yielded much exciting insight into the structure of the niche and the signalling pathways that it produces to regulate the self-renewal of stem cells. These findings are illuminating our understanding of the self-renewing mechanisms of tissue stem cells in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Weissman, I. L., Anderson, D. J. & Gage, F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell. Dev. Biol. 17, 387–403 (2001).
Spradling, A., Drummond-Barbosa, D. & Kai, T. Stem cells find their niche. Nature 414, 98–104 (2001).
Benfey, P. N. A tale of two kingdoms. Curr. Biol. 9, R171–R172 (1999).
Brown, E. H. & King, R. C. Oogonial and spermatogonial differentiation within a mosaic gonad of Drosophila melanogaster. Growth 26, 53–70 (1962).
Wieschaus, E. & Szabad, J. The development and function of the female germline in Drosophila melanogaster, a cell lineage study. Dev. Biol. 68, 29–46 (1979).
Lin, H. & Spradling, A. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Dev. Biol. 159, 140–152 (1993).
Drummond-Barbosa, D. & Spradling, A. C. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 231, 265–278 (2001).
King, F. J. & Lin, H. Somatic signaling mediated by fs(1)Yb is essential for germline stem cell maintenance during Drosophila oogenesis. Development 126, 1833–1844 (1999).
King, F. J., Szakmary, A., Cox, D. N. & Lin, H. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Mol. Cell 7, 497–508 (2001). References 8 and 9 report the first evidence that terminal filament and cap cells are essential for the maintenance of germline stem cells. Reference 9 is the first study to reveal how a common niche regulates the division of both germline and somatic stem cells.
Forbes, A. J., Lin, H., Ingham, P. W. & Spradling, A. C. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122, 1125–1135 (1996). This study indicates, for the first time, the possible existence of a signalling pathway that regulates stem cells in Drosophila.
Forbes, A. J., Spradling, A. C., Ingham, P. W. & Lin, H. The role of segment polarity genes during early oogenesis in Drosophila. Development 122, 3283–3294 (1996).
Cox, D. N. et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 12, 3715–3727 (1998). The discovery of the first and only known family of genes with stem-cell function that is highly conserved during evolution in both animal and plant kingdoms.
Cox, D. N., Chao, A. & Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127, 503–514 (2000).
Song, X., Zhu, C. H., Doan, C. & Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296, 1855–1857 (2002). The first genetic study showing the requirement for adherens junctions in stem-cell maintenance.
Xie, T. & Spradling, A. C. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94, 251–260 (1998). An elegant study showing the essential role of the dpp signalling pathway in germline stem-cell maintenance.
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000). This article provides a stringent criterion for defining a stem-cell niche.
Lin, H. & Spradling, A. C. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124, 2463–2476 (1997).
Deng, W. & Lin, H. Spectrosomes and fusomes are essential for anchoring mitotic spindles during asymmetric germ cell divisions and for the microtubule-based RNA transport during oocyte specification in Drosophila. Dev. Biol. 189, 79–94 (1997).
Bohmert, K. et al. AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 17, 170–180 (1998).
Moussian, B., Schoof, H., Haecker, A., Jurgens, G. & Laux, T. Role of the ZWILLE gene in the regulation of central shoot meristem cell fate during Arabidopsis embryogenesis. EMBO J. 17, 1799–1809 (1998).
Tabara, H. et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99, 123–132 (1999).
Cerutti, L., Mian, N. & Bateman, A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 25, 481–482 (2000).
Deng, W. & Lin, H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2, 819–830 (2002).
Tazuke, S. I. et al. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129, 2529–2539 (2002).
Hardy, R. W., Tokuyasu, K. T., Lindsley, D. L. & Garavito, M. The germinal proliferation center in the testis of Drosophila melanogaster. J. Ultrastruct. Res. 69, 180–190 (1979).
Gonczy, P. & DiNardo, S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development 122, 2437–2447 (1996).
Lin, H. The tao of stem cells in the germline. Annu. Rev. Genet. 31, 455–491 (1997).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science 294, 2546–2549 (2001). References 28 and 29 reveal the first signalling pathway from the testicular niche that is required for germline and somatic stem-cell division in Drosophila.
Hombría, J. C.-G. & Brown, S. The fertile field of Drosophila JAK/STAT signalling. Curr. Biol. 12, R569–R575 (2002).
McGregor, J. R., Xi, R. & Harrison, D. A. JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development 129, 705–717 (2002).
Matunis, E., Tran, J., Gonczy, P., Caldwell, K. & DiNardo, S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development 124, 4383–4391 (1997).
Tran, J., Brenner, T. J. & DiNardo, S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature 407, 754–757 (2000).
Kiger, A. A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000). References 33 and 34 define the role of Raf- and Egfr-mediated signalling pathways in restricting the proliferation of early germ cells in the testis.
Margolis, J. & Spradling, A. C. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 (1995).
Zhang, Y. & Kalderon, D. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604 (2001). An elegant study that characterizes the role of the Hh signalling pathway in regulating somatic stem-cell division.
Song, X. & Xie, T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl Acad. Sci. USA (in the press).
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Fuchs, E., Merrill, B. J., Jamora, C. & DasGupta, R. At the roots of a never-ending cycle. Dev. Cell 1, 13–25 (2001).
Oro, A. E. et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276, 817–821 (1997).
Besmer, P. The kit ligand encoded at the murine Steel locus: a pleiotropic growth and differentiation factor. Curr. Opin. Cell Biol. 3, 939–946 (1991).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Temple, S. The development of neural stem cells. Nature 414, 112–117 (2001).
Goldring, K., Partridge, T. & Watt, D. Muscle stem cells. J. Pathol. 197, 457–467 (2002).
Forbes, S., Vig, P., Poulsom, R., Thomas, H. & Alison, M. Hepatic stem cells. J. Pathol. 197, 510–518 (2002).
Gu, G., Dubauskaite, J. & Melton, D. A. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Zhao, G. Q., Deng, K., Labosky, P. A., Liaw, L. & Hogan, B. L. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 10, 1657–1669 (1996).
Matsuda, T. et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18, 4261–4269 (1999).
Ohta, H., Yomogida, K., Dohmae, K. & Nishimune, Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development 127, 2125–2131 (2000).
Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. 'Stemness': transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002).
Vanova, N. B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Gurdon, J. B. A community effect in animal development. Nature 336, 772–774 (1988).
Yang, L. et al. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc. Natl Acad. Sci. USA 99, 8078–8083 (2002).
Gussoni, E. et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390–394 (1999).
Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 (2002).
de Rooij, D. G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 121, 347–354 (2001).
Jones, L. Stem cells: so what's in a niche? Curr. Biol. 11, R484–R486 (2001).
Trenton, J. J. in Regulation of Hematopoietic Stem Cells (ed. Gordon, A. S.) 161–185 (Appleton–Century–Crofts, New York, 1970).
Potten, C. S. & Loeffler, M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110, 1001–1020 (1990).
Hall, P. A. & Watt, F. M. Stem cells: the generation and maintenance of cellular diversity. Development 106, 619–633 (1989).
Morrison, S. J., Shah, N. M. & Anderson, D. J. Regulatory mechanisms in stem cell biology. Cell 88, 287–298 (1997).
Lin, H., Yue, L. & Spradling, A. S. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development 120, 947–956 (1994).
Acknowledgements
I thank P. Bhattacharaya, M. Goddeeris, S. Grivna, M. Rivas, A. Szakmary and Z. Wang for their critical reading of the manuscript at extremely short notice. I also thank three anonymous reviewers for their comments. The writing of this review and the germline stem-cell research in my laboratory are supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Glossary
- TRANS-DIFFERENTIATION
-
The process by which stem or progenitor cells of one tissue give rise to the cell types of another tissue.
- STROMAL CELL
-
A connective tissue cell such as a fibroblast. In the stem-cell literature, this term often refers specifically to the connective cells that provide immediate support to a particular cell type.
- PAPILLA CELL
-
A stromal cell at the edge of the dermis that is in contact with the epidermis.
- CRYPT
-
The inpocketings on the lumenal surface of the small intestine.
- MOSAIC ANALYSIS
-
(also known as clonal analysis). The use of genetic or surgical operations to generate a genetically mosaic organism to examine the cell-autonomous (effect restricted to the cell in which it is expressed) versus cell-non-autonomous nature of a gene or developmental process.
- ADHERENS JUNCTION
-
A region of cell–cell adhesion that contains cadherin, which forms cell–cell junctions, and catenins, which anchor the actin skeleton to the plasma membrane.
- GERMARIUM
-
The apical, corn-shaped part of the insect ovariole (the functional unit of the insect ovary) that contains both germline and follicle stem cells and is responsible for the continuous production of new egg chambers.
- SIGNAL PEPTIDE
-
(or signal sequence). A short, 15–30-amino-acid sequence on a newly translated polypeptide that functions as a signal for its secretion from the cell. The signal peptide is removed as the protein is secreted.
- GAP JUNCTION
-
A type of junction between two cells through which ions and small molecules can pass.
Rights and permissions
About this article
Cite this article
Lin, H. The stem-cell niche theory: lessons from flies. Nat Rev Genet 3, 931–940 (2002). https://doi.org/10.1038/nrg952
Issue Date:
DOI: https://doi.org/10.1038/nrg952
This article is cited by
-
The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response
Nature Communications (2022)
-
Germline stem cells in human
Signal Transduction and Targeted Therapy (2022)
-
A focus on allogeneic mesenchymal stromal cells as a versatile therapeutic tool for treating multiple sclerosis
Stem Cell Research & Therapy (2021)
-
New insights into the role and origin of pituitary S100β-positive cells
Cell and Tissue Research (2021)
-
Perspective of placenta derived mesenchymal stem cells in acute liver failure
Cell & Bioscience (2020)