Abstract
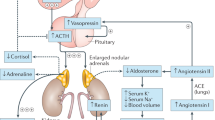
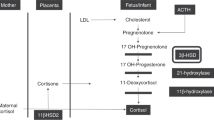
Pregnancy is marked by alterations in a number of endocrine systems, including activation of the renin–angiotensin–aldosterone system and the hypothalamic–pituitary–adrenal axis. The placenta, the fetal adrenal glands and the liver constitute an interactive endocrine entity, known as the fetoplacental unit. In the fetoplacental unit, the fetal adrenal glands are the primary source of dehydroepiandrosterone sulphate, which is further metabolized by the fetal liver and placenta to produce a variety of oestrogens. Several disorders can affect both the fetal and maternal adrenal glands during pregnancy. The most common fetal adrenal disorder, steroid 21-hydroxylase deficiency, leads to abnormalities in sexual development and can be life threatening for the neonate. Although rare, maternal adrenal disorders are associated with considerable maternal mortality and morbidity if not promptly recognized and treated. However, diagnosis is often difficult to establish because of the endocrine changes occurring during normal pregnancies and the lack of reference values for the majority of the adrenal steroids. This Review provides an overview of adrenal steroid metabolism during pregnancy and focuses on diagnosis and treatment of the most common fetal and maternal adrenal disorders.
Key Points
-
Adrenal disorders are rare in pregnancy but are associated with considerable maternal and fetal mortality and morbidity if not promptly diagnosed and treated
-
CYP21A2 deficiency is the most common cause of congenital adrenal hyperplasia, which causes fetal androgen excess and female fetus virilization; however, the placenta protects the mother against androgen exposure
-
The most frequent cause of Cushing syndrome during pregnancy is an adrenocortical adenoma; surgical treatment is recommended to improve fetal and maternal outcomes
-
Primary aldosteronism can improve spontaneously during pregnancy and is often successfully controlled with medical therapy
-
If pheochromocytoma is diagnosed during pregnancy, adrenalectomy should always be considered, as untreated pheochromocytoma is associated with maternal mortality as high as 48%
-
Adrenal insufficiency can be undiagnosed during pregnancy because circulating levels of cortisol increase twofold to threefold in pregnant women, such that plasma and urinary cortisol values within the nonpregnant normal range are misinterpreted as normal
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rainey, W. E., Rehman, K. S. & Carr, B. R. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin. Reprod. Med. 22, 327–336 (2004).
Ishimoto, H. & Jaffe, R. B. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr. Rev. 32, 317–355 (2011).
Diczfalusy, E. Endocrine functions of the human fetoplacental unit. Fed. Proc. 23, 791–798 (1964).
Lindsay, J. R. & Nieman, L. K. The hypothalamic–pituitary–adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr. Rev. 26, 775–799 (2005).
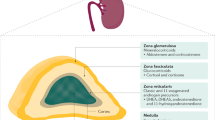
Mesiano, S., Coulter, C. L. & Jaffe, R. B. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17, 20-lyase, and 3 β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J. Clin. Endocrinol. Metab. 77, 1184–1189 (1993).
Ben-David, S. et al. Parturition itself is the basis for fetal adrenal involution. J. Clin. Endocrinol. Metab. 92, 93–97 (2007).
Goto, M. et al. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J. Clin. Invest. 116, 953–960 (2006).
Rehman, K. S., Carr, B. R. & Rainey, W. E. Profiling the steroidogenic pathway in human fetal and adult adrenals. J. Soc. Gynecol. Investig. 10, 372–380 (2003).
Siiteri, P. K. & MacDonald, P. C. Placental estrogen biosynthesis during human pregnancy. J. Clin. Endocrinol. Metab. 26, 751–761 (1966).
Bolte, E., Mancuso, S., Eriksson, G., Wiqvist, N. & Diczfalusy, E. Studies on the aromatisation of neutral steroids in pregnant women. I. Aromatisation of C-19 steroids by placentas perfused in situ. Acta Endocrinol. (Copenh.) 45, 535–559 (1964).
Pang, S. et al. Amniotic fluid concentrations of Δ5 and Δ4 steroids in fetuses with congenital adrenal hyperplasia due to 21 hydroxylase deficiency and in anencephalic fetuses. J. Clin. Endocrinol. Metab. 51, 223–229 (1980).
Buster, J. E. et al. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. I. C21 steroids: progesterone, 16α-hydroxyprogesterone, 17α-hydroxyprogesterone, 20α-dihydroprogesterone, Δ5-pregnenolone sulfate and 17-hydroxy Δ5-pregnenolone. J. Clin. Endocrinol. Metab. 48, 133–138 (1979).
Rouse, D. J. et al. A trial of 17 α-hydroxyprogesterone caproate to prevent prematurity in twins. N. Engl. J. Med. 357, 454–461 (2007).
Meis, P. J. et al. Prevention of recurrent preterm delivery by 17 α-hydroxyprogesterone caproate. N. Engl. J. Med. 348, 2379–2385 (2003).
Bocian-Sobkowska, J., Malendowicz, L. K., Wozniak, W. & Kopaczyk, M. Adrenal glands in anencephaly. Folia Morphol. (Warsz.) 55, 214–216 (1996).
Parker, C. R. Jr, Stankovic, A. M. & Goland, R. S. Corticotropin-releasing hormone stimulates steroidogenesis in cultured human adrenal cells. Mol. Cell Endocrinol. 155, 19–25 (1999).
Sirianni, R., Rehman, K. S., Carr, B. R., Parker, C. R. Jr & Rainey, W. E. Corticotropin-releasing hormone directly stimulates cortisol and the cortisol biosynthetic pathway in human fetal adrenal cells. J. Clin. Endocrinol. Metab. 90, 279–285 (2005).
Sirianni, R., Mayhew, B. A., Carr, B. R., Parker, C. R. Jr & Rainey, W. E. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J. Clin. Endocrinol. Metab. 90, 5393–5400 (2005).
Smith, R., Mesiano, S., Chan, E. C., Brown, S. & Jaffe, R. B. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J. Clin. Endocrinol. Metab. 83, 2916–2920 (1998).
Petraglia, F., Sawchenko, P. E., Rivier, J. & Vale, W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature 328, 717–719 (1987).
Goland, R. S., Wardlaw, S. L., Stark, R. I., Brown, L. S. Jr & Frantz, A. G. High levels of corticotropin-releasing hormone immunoactivity in maternal and fetal plasma during pregnancy. J. Clin. Endocrinol. Metab. 63, 1199–1203 (1986).
Florio, P., Woods, R. J., Genazzani, A. R., Lowry, P. J. & Petraglia, F. Changes in amniotic fluid immunoreactive corticotropin-releasing factor (CRF) and CRF-binding protein levels in pregnant women at term and during labor. J. Clin. Endocrinol. Metab. 82, 835–838 (1997).
Chrousos, G. P., Torpy, D. J. & Gold, P. W. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann. Intern. Med. 129, 229–240 (1998).
Robinson, B. G., Emanuel, R. L., Frim, D. M. & Majzoub, J. A. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc. Natl Acad. Sci. USA 85, 5244–5248 (1988).
Jones, S. A., Brooks, A. N. & Challis, J. R. Steroids modulate corticotropin-releasing hormone production in human fetal membranes and placenta. J. Clin. Endocrinol. Metab. 68, 825–830 (1989).
Bocian-Sobkowska, J., Malendowicz, L. K. & Wozniak, W. Comparative stereological study on zonation and cellular composition of adrenal glands of normal and anencephalic human fetuses. I. Zonation of the gland. Histol. Histopathol. 12, 311–317 (1997).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157 (1998).
Jackson, R. S. et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J. Clin. Invest. 112, 1550–1560 (2003).
Clark, A. J., McLoughlin, L. & Grossman, A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341, 461–462 (1993).
Metherell, L. A. et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 (2005).
Metherell, L. A., Chan, L. F. & Clark, A. J. The genetics of ACTH resistance syndromes. Best Pract. Res. Clin. Endocrinol. Metab. 20, 547–560 (2006).
Metherell, L. A. et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 94, 3865–3871 (2009).
Hughes, C. R. et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 122, 814–820 (2012).
Meimaridou, E. et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat. Genet. 44, 740–742 (2012).
Parker, K. L. et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog. Horm. Res. 57, 19–36 (2002).
Hanley, N. A., Rainey, W. E., Wilson, D. I., Ball, S. G. & Parker, K. L. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol. Endocrinol. 15, 57–68 (2001).
Peter, M., Viemann, M., Partsch, C. J. & Sippell, W. G. Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J. Clin. Endocrinol. Metab. 83, 2666–2674 (1998).
Shen, W. H., Moore, C. C., Ikeda, Y., Parker, K. L. & Ingraham, H. A. Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell 77, 651–661 (1994).
Achermann, J. C., Ito, M., Ito, M., Hindmarsh, P. C. & Jameson, J. L. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 22, 125–126 (1999).
El-Khairi, R., Martinez-Aguayo, A., Ferraz-de-Souza, B., Lin, L. & Achermann, J. C. Role of DAX-1 (NR0B1) and steroidogenic factor-1 (NR5A1) in human adrenal function. Endocr. Dev. 20, 38–46 (2011).
Schimmer, B. P. & White, P. C. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol. Endocrinol. 24, 1322–1337 (2010).
Speiser, P. W. et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 95, 4133–4160 (2010).
Lekarev, O., Mallet, D., Yuen, T., Morel, Y. & New, M. I. Congenital lipoid adrenal hyperplasia (a rare form of adrenal insufficiency and ambiguous genitalia) caused by a novel mutation of the steroidogenic acute regulatory protein gene. Eur. J. Pediatr. 171, 787–793 (2012).
Hauffa, B. & Hiort, O. P450 side-chain cleavage deficiency--a rare cause of congenital adrenal hyperplasia. Endocr. Dev. 20, 54–62 (2011).
Krone, N. & Arlt, W. Genetics of congenital adrenal hyperplasia. Best Pract. Res. Clin. Endocrinol. Metab. 23, 181–192 (2009).
Nimkarn, S. & New, M. I. Prenatal diagnosis and treatment of congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Nat. Clin. Pract. Endocrinol. Metab. 3, 405–413 (2007).
New, M. I. et al. Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J. Clin. Endocrinol. Metab. 86, 5651–5657 (2001).
Lajic, S., Nordenström, A. & Hirvikoski, T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocr. Dev. 20, 96–105 (2011).
Mercè Fernández-Balsells, M. et al. Prenatal dexamethasone use for the prevention of virilization in pregnancies at risk for classical congenital adrenal hyperplasia because of 21-hydroxylase (CYP21A2) deficiency: a systematic review and meta-analyses. Clin. Endocrinol. (Oxf.) 73, 436–444 (2010).
Reisch, N. et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 94, 1665–1670 (2009).
Hagenfeldt, K. et al. Fertility and pregnancy in outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum. Reprod. 23, 1607–1613 (2008).
Casteràs, A., De Silva, P., Rumsby, G. & Conway, G. S. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin. Endocrinol. (Oxf.) 70, 833–837 (2009).
Lo, J. C. et al. Normal female infants born of mothers with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 84, 930–936 (1999).
Bidet, M. et al. Fertility in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 95, 1182–1190 (2010).
Flück, C. E. et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley–Bixler syndrome. Nat. Genet. 36, 228–230 (2004).
Craig, W. Y. et al. Prevalence of steroid sulfatase deficiency in California according to race and ethnicity. Prenat. Diagn. 30, 893–898 (2010).
Taylor, N. F. Review: placental sulphatase deficiency. J. Inherit. Metab. Dis. 5, 164–176 (1982).
Hauri-Hohl, A. et al. Aromatase deficiency due to a functional variant in the placenta promoter and a novel missense mutation in the CYP19A1 gene. Clin. Endocrinol. (Oxf.) 75, 39–43 (2011).
Morishima, A., Grumbach, M. M., Simpson, E. R., Fisher, C. & Qin, K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 80, 3689–3698 (1995).
Dörr, H. G. et al. Longitudinal study of progestins, mineralocorticoids, and glucocorticoids throughout human pregnancy. J. Clin. Endocrinol. Metab. 68, 863–868 (1989).
Landau, R. L. & Lugibihl, K. The catabolic and natriuretic effects of progesterone in man. Recent Prog. Horm. Res. 17, 249–292 (1961).
Parker, C. R., Jr., Cutrer, S., Casey, M. L. & MacDonald, P. C. Concentrations of deoxycorticosterone, deoxycorticosterone sulfate, and progesterone in maternal venous serum and umbilical arterial and venous sera. Am. J. Obstet. Gynecol. 145, 427–432 (1983).
Casey, M. L. & MacDonald, P. C. Extraadrenal formation of a mineralocorticosteroid: deoxycorticosterone and deoxycorticosterone sulfate biosynthesis and metabolism. Endocr. Rev. 3, 396–403 (1982).
Carr, B. R., Parker, C. R. Jr, Madden, J. D., MacDonald, P. C. & Porter, J. C. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am. J. Obstet. Gynecol. 139, 416–422 (1981).
Burke, C. W. Biologically active cortisol in plasma of oestrogen-treated and normal subjects. Br. Med. J. 2, 798–800 (1969).
Sasaki, A. et al. Immunoreactive corticotropin-releasing hormone present in human plasma may be derived from both hypothalamic and extrahypothalamic sources. J. Clin. Endocrinol. Metab. 65, 176–182 (1987).
Baulieu, E. E. & Dray, F. Conversion of H3-dehydroisoandrosterone (3β-hydroxy-Δ5-androsten-17-one) sulfate to H3-estrogens in normal pregnant women. J. Clin. Endocrinol. Metab. 23, 1298–1301 (1963).
Bélisle, S., Schiff, I. & Tulchinsky, D. The use of constant infusion of unlabeled dehydroepiandrosterone for the assessment of its metabolic clearance rate, its half-life, and its conversion into estrogens. J. Clin. Endocrinol. Metab. 50, 117–121 (1980).
Crane, M. G., Andes, J. P., Harris, J. J. & Slate, W. G. Primary aldosteronism in pregnancy. Obstet. Gynecol. 23, 200–208 (1964).
Ananth, C. V., Peltier, M. R., Kinzler, W. L., Smulian, J. C. & Vintzileos, A. M. Chronic hypertension and risk of placental abruption: is the association modified by ischemic placental disease? Am. J. Obstet. Gynecol. 197, 273.e1–273.e7 (2007).
Sibai, B. M. Chronic hypertension in pregnancy. Obstet. Gynecol. 100, 369–377 (2002).
Chappell, L. C. et al. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: a prospective study. Hypertension 51, 1002–1009 (2008).
Ronconi, V., Turchi, F., Zennaro, M. C., Boscaro, M. & Giacchetti, G. Progesterone increase counteracts aldosterone action in a pregnant woman with primary aldosteronism. Clin. Endocrinol. (Oxf.) 74, 278–279 (2011).
Biglieri, E. G. & Slaton, P. E. Jr. Pregnancy and primary aldosteronism. J. Clin. Endocrinol. Metab. 27, 1628–1632 (1967).
Aoi, W. et al. Primary aldosteronism aggravated during peripartum period. Jpn Heart J. 19, 946–953 (1978).
Gordon, R. D. & Tunny, T. J. Aldosterone-producing-adenoma (A-P-A): effect of pregnancy. Clin. Exp. Hypertens. A 4, 1685–1693 (1982).
Murakami, T., Watanabe Ogura, E., Tanaka, Y. & Yamamoto, M. High blood pressure lowered by pregnancy. Lancet 356, 1980 (2000).
Quinkler, M., Meyer, B., Oelkers, W. & Diederich, S. Renal inactivation, mineralocorticoid generation, and 11beta-hydroxysteroid dehydrogenase inhibition ameliorate the antimineralocorticoid effect of progesterone in vivo. J. Clin. Endocrinol. Metab. 88, 3767–3772 (2003).
Albiger, N. M. et al. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur. J. Endocrinol. 164, 405–412 (2011).
Wyckoff, J. A. et al. Glucocorticoid-remediable aldosteronism and pregnancy. Hypertension 35, 668–672 (2000).
Funder, J. et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 3266–3281 (2008).
Messina, M., Biffignandi, P., Ghigo, E., Jeantet, M. G. & Molinatti, G. M. Possible contraindication of spironolactone during pregnancy. J. Endocrinol. Invest. 2, 222 (1979).
Groves, T. D. & Corenblum, B. Spironolactone therapy during human pregnancy. Am. J. Obstet. Gynecol. 172, 1655–1656 (1995).
Regitz-Zagrosek, V. et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 32, 3147–3197 (2011).
Al-Ali, N. A., El-Sandabesee, D., Steel, S. A. & Roland, J. M. Conn's syndrome in pregnancy successfully treated with amiloride. J. Obstet. Gynaecol 27, 730–731 (2007).
Cabassi, A., Rocco, R., Berretta, R., Regolisti, G. & Bacchi-Modena, A. Eplerenone use in primary aldosteronism during pregnancy. Hypertension 59, e18–e19 (2012).
Nieman, L. K. et al. The diagnosis of Cushing's syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 1526–1540 (2008).
Lado-Abeal, J. et al. Menstrual abnormalities in women with Cushing's disease are correlated with hypercortisolemia rather than raised circulating androgen levels. J. Clin. Endocrinol. Metab. 83, 3083–3088 (1998).
Buescher, M. A., McClamrock, H. D. & Adashi, E. Y. Cushing syndrome in pregnancy. Obstet. Gynecol. 79, 130–137 (1992).
Wallace, C., Toth, E. L., Lewanczuk, R. Z. & Siminoski, K. Pregnancy-induced Cushing's syndrome in multiple pregnancies. J. Clin. Endocrinol. Metab. 81, 15–21 (1996).
Close, C. F., Mann, M. C., Watts, J. F. & Taylor, K. G. ACTH-independent Cushing's syndrome in pregnancy with spontaneous resolution after delivery: control of the hypercortisolism with metyrapone. Clin. Endocrinol. (Oxf.) 39, 375–379 (1993).
Chui, M. H. et al. Case report: Adrenal LH/hCG receptor overexpression and gene amplification causing pregnancy-induced Cushing's syndrome. Endocr. Pathol. 20, 256–261 (2009).
Lacroix, A., Ndiaye, N., Tremblay, J. & Hamet, P. Ectopic and abnormal hormone receptors in adrenal Cushing's syndrome. Endocr. Rev. 22, 75–110 (2001).
Rask, E. et al. Adrenocorticotropin-independent Cushing's syndrome in pregnancy related to overexpression of adrenal luteinizing hormone/human chorionic gonadotropin receptors. J. Endocrinol. Invest. 32, 313–316 (2009).
Wy, L. A. et al. Pregnancy-associated Cushing's syndrome secondary to a luteinizing hormone/human chorionic gonadotropin receptor-positive adrenal carcinoma. Gynecol. Endocrinol. 16, 413–417 (2002).
Lindsay, J. R., Jonklaas, J., Oldfield, E. H. & Nieman, L. K. Cushing's syndrome during pregnancy: personal experience and review of the literature. J. Clin. Endocrinol. Metab. 90, 3077–3083 (2005).
Pricolo, V. E. et al. Management of Cushing's syndrome secondary to adrenal adenoma during pregnancy. Surgery 108, 1072–1077 (1990).
Erichsen, M. M., Husebye, E. S., Michelsen, T. M., Dahl, A. A. & Løvås, K. Sexuality and fertility in women with Addison's disease. J. Clin. Endocrinol. Metab. 95, 4354–4360 (2010).
Björnsdottir, S. et al. Addison's disease in women is a risk factor for an adverse pregnancy outcome. J. Clin. Endocrinol. Metab. 95, 5249–5257 (2010).
Winqvist, O., Gustafsson, J., Rorsman, F., Karlsson, F. A. & Kämpe, O. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison's disease. J. Clin. Invest. 92, 2377–2385 (1993).
Albert, E., Dalaker, K., Jorde, R. & Berge, L. N. Addison's disease and pregnancy. Acta Obstet. Gynecol. Scand. 68, 185–187 (1989).
Ambrosi, B., Barbetta, L. & Morricone, L. Diagnosis and management of Addison's disease during pregnancy. J. Endocrinol. Invest. 26, 698–702 (2003).
Fux Otta, C., Szafryk de Mereshian, P., Iraci, G. S. & Ojeda de Pruneda, M. R. Pregnancies associated with primary adrenal insufficiency. Fertil. Steril. 90, 1199.e17–1199.e20 (2008).
Grinspoon, S. K. & Biller, B. M. Clinical review 62: Laboratory assessment of adrenal insufficiency. J. Clin. Endocrinol. Metab. 79, 923–931 (1994).
Suri, D., Moran, J., Hibbard, J. U., Kasza, K. & Weiss, R. E. Assessment of adrenal reserve in pregnancy: defining the normal response to the adrenocorticotropin stimulation test. J. Clin. Endocrinol. Metab. 91, 3866–3872 (2006).
Drucker, D., Shumak, S. & Angel, A. Schmidt's syndrome presenting with intrauterine growth retardation and postpartum addisonian crisis. Am. J. Obstet. Gynecol. 149, 229–230 (1984).
Lenders, J. W., Eisenhofer, G., Mannelli, M. & Pacak, K. Phaeochromocytoma. Lancet 366, 665–675 (2005).
Lenders, J. W. Pheochromocytoma and pregnancy: a deceptive connection. Eur. J. Endocrinol. 166, 143–150 (2012).
Schenker, J. G. & Chowers, I. Pheochromocytoma and pregnancy. Review of 89 cases. Obstet. Gynecol. Surv. 26, 739–747 (1971).
Ahlawat, S. K., Jain, S., Kumari, S., Varma, S. & Sharma, B. K. Pheochromocytoma associated with pregnancy: case report and review of the literature. Obstet. Gynecol. Surv. 54, 728–737 (1999).
Desai, A. S., Chutkow, W. A., Edelman, E., Economy, K. E. & Dec, G. W. Jr. A crisis in late pregnancy. N. Engl. J. Med. 361, 2271–2277 (2009).
Hudsmith, J. G., Thomas, C. E. & Browne, D. A. Undiagnosed phaeochromocytoma mimicking severe preeclampsia in a pregnant woman at term. Int. J. Obstet. Anesth. 15, 240–245 (2006).
Newell, K. A. et al. Pheochromocytoma multisystem crisis. A surgical emergency. Arch. Surg. 123, 956–959 (1988).
Lentschener, C., Gaujoux, S., Tesniere, A. & Dousset, B. Point of controversy: perioperative care of patients undergoing pheochromocytoma removal-time for a reappraisal? Eur. J. Endocrinol. 165, 365–373 (2011).
Mannelli, M. & Bemporad, D. Diagnosis and management of pheochromocytoma during pregnancy. J. Endocrinol. Invest. 25, 567–571 (2002).
Natrajan, P. G., McGarrigle, H. H., Lawrence, D. M. & Lachelin, G. C. Plasma noradrenaline and adrenaline levels in normal pregnancy and in pregnancy-induced hypertension. Br. J. Obstet. Gynaecol 89, 1041–1045 (1982).
Zuspan, F. P. Urinary excretion of epinephrine and norepinephrine during pregnancy. J. Clin. Endocrinol. Metab. 30, 357–360 (1970).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article (researching data for the article, discussion of the content, writing the manuscript, and review or editing of the manuscript before submission).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Adrenal-related hormone levels in pregnant and non-pregnant women (DOC 46 kb)
Rights and permissions
About this article
Cite this article
Monticone, S., Auchus, R. & Rainey, W. Adrenal disorders in pregnancy. Nat Rev Endocrinol 8, 668–678 (2012). https://doi.org/10.1038/nrendo.2012.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.155
This article is cited by
-
Practical consensus for the treatment and follow-up of primary aldosteronism: a multidisciplinary consensus document
Endocrine (2024)
-
Primary aldosteronism in pregnancy
Reviews in Endocrine and Metabolic Disorders (2023)
-
Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis
Reproductive Biology and Endocrinology (2015)