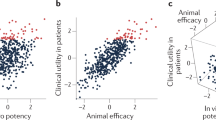
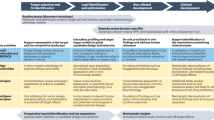
Abstract
New medicines are designed to bind to receptors or enzymes and are tested in animal cells, tissues and whole organisms in a highly scientific process. Subsequently they are often administered to human subjects with tolerability as the primary objective. The process of development is considered to be linear and consecutive and passes through the famous four phases of development (Phase I– Phase IV). This is efficient for those projects for which the uncertainty about the development is low. There is, however, an increasing number of new prototypical compounds resulting from the increased biological knowledge with a high level of uncertainty. For these prototypical drugs development has to proceed in a much more adaptive manner, using tailor-made objectives, the development of special methodology and a cyclical rather than a linear type of project management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
William Withering. An Account of the Foxglove and its Medical Uses (Oxford Univ. Press, London, 1785).
Ng, R. Drugs. From discovery to approval 2nd edn (John Wiley & Sons, Hoboken, New Jersey, 2009).
Rang, H. P. Drug Discovery and Development. 1st edn (Churchill Livingstone, Elsevier, Philadelphia, 2007).
Cross, J. et al. Postmarketing drug dosage changes of 499 FDA-approved new molecular entities, 1980–1999. Pharmacoepidemiol. Drug Saf. 11, 439–446 (2002).
Bhogal, N. & Combes, R. TGN1412: time to change the paradigm for the testing of new pharmaceuticals. Altern. Lab. Anim. 34, 225–239 (2006).
Clark, R. W. et al. Raising high-density lipoprotein in humans through inhibition of cholesteryl ester transfer protein: an initial multidose study of torcetrapib. Arterioscler. Thromb. Vasc. Biol. 24, 490–497 (2004).
Zhao, L., Jin, W., Rader, D., Packard, C. & Feuerstein, G. A translational medicine perspective of the development of torcetrapib: does the failure of torcetrapib development cast a shadow on future development of lipid modifying agents, HDL elevation strategies or CETP as a viable molecular target for atherosclerosis? A case study of the use of biomarkers and translational medicine in atherosclerosis drug discovery and development. Biochem. Pharmacol. 78, 315–325 (2009).
US Food and Drug Administration. Note for guidance on general considerations for clinical trials. Fed. Regist. 62(242), 66113–66119 (1997).
Lenfle, S. & Loch, C. Lost roots. How project management settled on the phased approach (and compromised its ability to lead change in modern enterprises). Ecole Polytechnique website [online], (2009).
Loch, C., DeMeyer, A. & Pich, M. Managing the unknown. A new approach to managing high uncertainty and risk in projects. (John Wiley & Sons, Hoboken, New Jersey, 2006).
Shuchman, M. Commercializing clinical trials-risks and benefits of the CRO boom. N. Engl. J. Med. 357, 1365–1368 (2007).
Bresalier, R. S. et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 352, 1092–1102 (2005).
Garnier, J. P. Rebuilding the R&D engine in big pharma. Harv. Bus Rev. 86, 68–70, 72–76, 128 (2008).
Cuatrecasas, P. Drug discovery in jeopardy. J. Clin. Invest. 116, 2837–2842 (2006).
Munos, B. Lessons from 60 years of pharmaceutical innovation. Nature Rev. Drug Discov. 8, 959–968 (2009).
Lehman Brothers. The fruits of genomics. Drug pipelines face indigestion until the new biology ripens. (Lehman Brothers, New York, 2001).
US Food and Drug Administration. Challenge and opportunity on the critical path to new medical products. FDA website [online], (2004).
Loch, C., Mihm, J. & Huchzermeier, A. Concurrent engineering and design oscillations in complex engineering projects. Concurrent Eng. 11, 187–199 (2003).
Allen, T., Thusman, M. & Lee, D. Technology transfer as a function of position in the spectrum from research through development to technical services. Acad. Manage. J. 22, 694–708 (1979).
Roussel P. A., Saad, K. N. & Erickson, T. J. Third generation R&D: Managing the Link to Corporate Strategy 1st edn (Harvard Bus School Press, Boston, Massachusetts, USA, 1991).
Sheiner, L. B. Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 61, 275–291 (1997).
Danhof, M., Alvan, G., Dahl, S. G., Kuhlmann, J. & Paintaud, G. Mechanism-based pharmacokinetic–pharmacodynamic modeling-a new classification of biomarkers. Pharm. Res. 22, 1432–1437 (2005).
FDA Center for Drug Evaluation and Research. Exploratory I.N.D. Studies. FDA website [online], (2006).
Kenter, M. J. & Cohen, A. F. Establishing risk of human experimentation with drugs: lessons from TGN1412. Lancet 368, 1387–1391 (2006).
Cohen, A. Pharmacokinetic and pharmacodynamic data to be derived from early-phase drug development: designing informative human pharmacology studies. Clin. Pharmacokinet. 47, 373–381 (2008).
FDA Center for Drug Evaluation and Research. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Guidance for Industry. FDA website [online], (2005).
Cohen, A. Should we tolerate tolerability as an objective in early drug development? Br. J. Clin. Pharmacol. 64, 249–252 (2007).
Schein, P. S. et al. The evaluation of anticancer drugs in dogs and monkeys for the prediction of qualitative toxicities in man. Clin. Pharmacol. Ther. 11, 3–40 (1970).
Freireich, E. J., Gehan, E. A., Rall, D. P., Schmidt, L. H. & Skipper, H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother. Rep. 50, 219–244 (1966).
Zhou, H. et al. Effect of meal timing not critical for the pharmacokinetics of tegaserod (HTF 919). J. Clin. Pharmacol. 39, 911–919 (1999).
Appel, S., Kumle, A., Hubert, M. & Duvauchelle, T. First pharmacokinetic–pharmacodynamic study in humans with a selective 5-hydroxytryptamine4 receptor agonist. J. Clin. Pharmacol. 37, 229–237 (1997).
Chan K. Y. et al. Functional characterization of contractions to tegaserod in human isolated proximal and distal coronary arteries. Eur. J. Pharmacol. 619, 61–67 (2009).
Farzaneh, L., Kasahara, N. & Farzaneh, F. The strange case of TGN1412. Cancer Immunol. Immunother. 56, 129–134 (2007).
Legrand, N. et al. Transient accumulation of human mature thymocytes and regulatory T cells with CD28 superagonist in “human immune system” Rag2(−/−)γc(−/−) mice. Blood 108, 238–245 (2006).
Expert Scientific Group on Phase One clinical Trials. Final Report (The Stationary Office, Norwich, UK, 2006).
de Visser, S. J. et al. Concentration-effect relationships of two rilmenidine single-dose infusion rates in hypertensive patients. Clin. Pharmacol. Ther. 72, 419–428 (2002).
van der Post, J. P., de Visser, S. J., Schoemaker, R. C., Cohen, A. F. & van Gerven, J. M. Pharmacokinetic/pharmacodynamic assessment of tolerance to central nervous system effects of a 3 mg sustained release tablet of rilmenidine in hypertensive patients. J. Psychopharmacol. 18, 221–227 (2004).
de Visser, S. J. et al. Biomarkers for the effects of benzodiazepines in healthy volunteers. Br. J. Clin. Pharmacol. 55, 39–50 (2003).
Brisbare-Roch, C. et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nature Med. 13, 150–155 (2007).
de Visser, S. J. A question based approach to drug development. Thesis, Leiden Univ. (2003).
Aronson, J. K. & Ferner, R. E. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ 327, 1222–1225 (2003).
Franson, K. L. & Cohen, A. F. How it works. Br. J. Clin. Pharmacol. 68, 315–317 (2009).
Brunton, L., Blumenthal, D., Buxton, I. & Parker, K. Goodman and Gilman's Manual of Pharmacology and Therapeutics (McGraw Hill Medical, USA, 2006).
Dollery, C. T. Clinical pharmacology in the molecular era. Clin. Pharmacol. Ther. 83, 220–225 (2008).
Aronson, J. An account of the foxglove and its medical uses, 1785–1985. (Oxford Univ. Pres, London 1985). Clin. Pharmacol. Ther. 83, 220–225 (2008).
van Gerven, J. M. et al. Integrated pharmacokinetics and pharmacodynamics of Ro 48–8684, a new benzodiazepine, in comparison with midazolam during first administration to healthy male subjects. Br. J. Clin. Pharmacol. 44, 487–493 (1997).
Acknowledgements
I am indebted to my close colleagues at the Centre for Human Drug Research, Leiden, The Netherlands, particularly J. van Gerven and K. Burggraaf, for their input in the ideas that form the basis for this article. M. Kenter, D. Breimer and P. van Brummelen provided constructive criticism and suggestions.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Cohen, A. Developing drug prototypes: pharmacology replaces safety and tolerability?. Nat Rev Drug Discov 9, 856–865 (2010). https://doi.org/10.1038/nrd3227
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3227
This article is cited by
-
EEG machine learning for accurate detection of cholinergic intervention and Alzheimer’s disease
Scientific Reports (2017)
-
Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm
European Journal of Clinical Pharmacology (2017)
-
Animal models and conserved processes
Theoretical Biology and Medical Modelling (2012)
-
Translating Discoveries into Medicine: Psychiatric Drug Development in 2011
Neuropsychopharmacology (2012)
-
Portfolio Decisions in Early Development
Pharmaceutical Medicine (2012)