Abstract
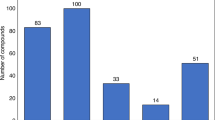
The economic effects of the possible introduction of 'follow-on' protein products have been the subject of recent debate. Here, we aim to explore the economic issues surrounding this debate using three measures: total sales, product complexity and patent expiry. Our analysis shows that the sales of therapeutic protein products are concentrated in a relatively small number of branded products, which may be the most attractive targets for follow-on development. For the years 2013–2015, we estimate that products representing US$20 billion in annual sales — approximately half of all sales in 2006 — can be expected to lose patent protection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Express Scripts. Press release 25 April. Biotech Drug Spending Increases 21 Percent Even as Growth in Rx Expenditure Slows. Express Scripts web site [online], (2007).
IMS Health. IMS National Sales Perspectives: Retail and Non-Retail Combined Purchases, January–December 2006 (2007).
Bhattycharyya, L. et al. Equivalence studies for complex active ingredients and dosage forms, matrix of protein types. AAPS J. 7, e786–e812 (2005).
Sheridan, C. First generic biologics finally approved. Nature Rev. Drug Discov. 5, 445 (2006).
Aggarwal, S. What's fueling the biotech engine? Nature Biotech. 25, 1097–1104 (2007).
Manheim, B. S. Jr, Granahan, P. & Dow, K. J. 'Follow-on biologics': ensuring continued innovation in the biotechnology industry. Health Aff. 25, 394–404 (2006).
Biotechnology Industry Organization (BIO). A Brief Primer on Manufacturing Therapeutic Proteins. BIO web site [online], (2002).
Grabowski, H. G. Statement before Committee on Oversight and Government Reform (COGR), United States House of Representatives. COGR web site [online], (2007).
Grabowski, H. G., Cockburn, I. & Long, G. The market for follow-on biologics: how will it evolve? Health Aff. 25, 1291–1301 (2006).
Woodcock, J. et al. The FDA's assessment of follow-on protein products: a historical perspective. Nature Rev. Drug Discov. 6, 437–442 (2007).
Woodcock, J. Statement before Committee on Oversight and Government Reform (COGR), United States House of Representatives. COGR web site [online], (2007).
Engel & Novitt, LLP . Potential Savings That Might Be Realized By the Medicare Program From Enactment Of Legislation Such As The Access To Life-Saving Medicine Act (H.R.6257/S.4016) That Establishes A New cBLA Pathway For Follow-On Biologics. A Report To Pharmaceutical Care Management Association (PCMA) Based Upon A Preliminary Assessment Of Available Data. PCMA web site[online], (2007).
Miller, S. & Houts, J. Potential Savings of Biogenerics in The United States. Express Scripts web site [online], (2007).
Ahlstrom, A., King, R., Brown, R., Glaudemans, J. & Mendelson, D. Modeling Federal Cost Savings from Follow-On Biologics. Alvare Health web site [online], (2007).
Congressional Budget Office Cost Estimate. S. 1695 Biologics Price Competition and Innovation Act of 2007. CBO website [online], (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lanthier, M., Behrman, R. & Nardinelli, C. Economic issues with follow-on protein products. Nat Rev Drug Discov 7, 733–737 (2008). https://doi.org/10.1038/nrd2636
Issue Date:
DOI: https://doi.org/10.1038/nrd2636
This article is cited by
-
Analytical techniques and bioactivity assays to compare the structure and function of filgrastim (granulocyte-colony stimulating factor) therapeutics from different manufacturers
Analytical and Bioanalytical Chemistry (2014)
-
Demonstration of Biosimilarity, Extrapolation of Indications and Other Challenges Related to Biosimilars in Europe
BioDrugs (2014)
-
Development and Regulation of Biosimilars: Current Status and Future Challenges
BioDrugs (2013)
-
Strategies and challenges for the next generation of therapeutic antibodies
Nature Reviews Immunology (2010)
-
Biosimilars: Evidential Standards for Health Technology Assessment
Clinical Pharmacology & Therapeutics (2010)