Abstract
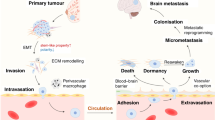
Brain metastases are a serious obstacle in the treatment of patients with solid tumors and contribute to the morbidity and mortality of these cancers. It is speculated that the frequency of brain metastasis is increasing for several reasons, including improved systemic therapy and survival, and detection of metastases in asymptomatic patients. The lack of preclinical models that recapitulate the clinical setting and the exclusion of patients with brain metastases from most clinical trials have slowed progress. Molecular factors contributing to brain metastases are being elucidated, such as genes involved in cell adhesion, extravasation, metabolism, and cellular signaling. Furthermore, the role of the unique brain microenvironment is beginning to be explored. Although the presence and function of the blood–brain barrier in metastatic tumors is still poorly understood, it is likely that some tumor cells are protected from therapeutics by the blood–tumor barrier, creating a sanctuary site. This Review discusses what is known about the biology of brain metastases, what preclinical models are available to study the disease, and which novel therapeutic strategies are being studied in patients.
Key Points
-
Longer survival of patients and more-sensitive detection of metastatic disease by improved imaging modalities might contribute to the increased incidence of detected brain metastases
-
Novel preclinical models that more accurately represent clinical brain metastases and imaging techniques that allow study of the formation of brain metastases and their response to treatments are emerging
-
Brain metastases grow by co-opting existing blood vessels and/or by forming new blood vessels; the brain microenvironment promotes tumor cell survival, tumor growth and resistance to therapy
-
Although a lesser problem in large metastases, the blood–brain barrier (BBB) could prevent therapeutic access to micrometastases; strategies to enhance drug delivery across the BBB are under investigation
-
Differential expression of genes involved in intravasation and extravasation, metabolism, cell adhesion, and cellular signaling in brain-specific metastatic clones have been identified
-
Targeted therapies, including inhibitors of EGFR, HER2, PI3K and BRAF, have shown promise in the treatment of brain metastases, but require testing in randomized trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gavrilovic, I. T. & Posner, J. B. Brain metastases: epidemiology and pathophysiology. J. Neurooncol. 75, 5–14 (2005).
Barnholtz-Sloan, J. S. et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 22, 2865–2872 (2004).
Schouten, L. J., Rutten, J., Huveneers, H. A. & Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94, 2698–2705 (2002).
Delattre, J. Y., Krol, G., Thaler, H. T. & Posner, J. B. Distribution of brain metastases. Arch. Neurol. 45, 741–744 (1988).
Chang, E. L. et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 60, 277–283 (2007).
Mehta, M. P. et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J. Clin. Oncol. 21, 2529–2536 (2003).
Gaspar, L. et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 37, 745–751 (1997).
Melisko, M. E., Moore, D. H., Sneed, P. K., De Franco, J. & Rugo, H. S. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J. Neurooncol. 88, 359–365 (2008).
Eichler, A. F. et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 112, 2359–2367 (2008).
Eichler, A. F. et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 12, 1193–1199 (2010).
Patchell, R. A. et al. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 322, 494–500 (1990).
Vecht, C. J. et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann. Neurol. 33, 583–590 (1993).
Andrews, D. W. et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363, 1665–1672 (2004).
Aoyama, H. et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295, 2483–2491 (2006).
Kocher, M. et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J. Clin. Oncol. 29, 134–141 (2011).
Antonadou, D. et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J. Clin. Oncol. 20, 3644–3650 (2002).
Robinet, G. et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) Protocol 95–1. Ann. Oncol. 12, 59–67 (2001).
Verger, E. et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 61, 185–191 (2005).
Talmadge, J. E. & Fidler, I. J. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 70, 5649–5669 (2010).
Hess, K. R. et al. Metastatic patterns in adenocarcinoma. Cancer 106, 1624–33 (2006).
Patel, J. K., Didolkar, M. S., Pickren, J. W. & Moore, R. H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 135, 807–10 (1978).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Ewing, J. Neoplastic Diseases. A Treatise on Tumors (W. B. Saunders Co., Philadelphia and London, 1928).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Lorger, M. & Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 176, 2958–2971 (2010).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, e5857 (2009).
Fitzgerald, D. P. et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metastasis 25, 799–810 (2008).
Zhang, M. & Olsson, Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J. Neurol. Sci. 134, 26–32 (1995).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer 9, 239–252 (2009).
Pukrop, T. et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58, 1477–1489 (2010).
He, B. P. et al. Differential reactions of microglia to brain metastasis of lung cancer. Mol. Med. 12, 161–170 (2006).
Fidler, I. J. The role of the organ microenvironment in brain metastasis. Semin. Cancer Biol. 21, 107–112 (2011).
Langley, R. R. et al. Generation of an immortalized astrocyte cell line from H-2Kb-tsA58 mice to study the role of astrocytes in brain metastasis. Int. J. Oncol. 35, 665–672 (2009).
Lin, Q. et al. Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels. Neoplasia 12, 748–754 (2010).
Kim, S. J. et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy Neoplasia 13, 286–298 (2011).
Seike, T. et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin. Exp. Metastasis 28, 13–25 (2010).
Denkins, Y. et al. Brain metastases in melanoma: roles of neurotrophins. Neuro Oncol. 6, 154–165 (2004).
Menter, D. G., Herrmann, J. L. & Nicolson, G. L. The role of trophic factors and autocrine/paracrine growth factors in brain metastasis. Clin. Exp. Metastasis 13, 67–88 (1995).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 6, 273–286 (2007).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature (in press).
Jain, R. K. et al. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 8, 610–622 (2007).
Ricci-Vitiani, L. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010).
Wang, R. et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468, 829–833 (2010).
Di Tomaso, E. et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 71, 19–28 (2011).
Soda, Y. et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl Acad. Sci. USA 108, 4274–4280 (2011).
Kusters, B. et al. Vascular endothelial growth factor-A165 induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 62, 341–345 (2002).
Leenders, W. P. et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin. Cancer Res. 10, 6222–6230 (2004).
Fidler, I. J., Yano, S., Zhang, R. D., Fujimaki, T. & Bucana, C. D. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 3, 53–57 (2002).
Yano, S. et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 60, 4959–4967 (2000).
Kim, L. S., Huang, S., Lu, W., Lev, D. C. & Price, J. E. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin. Exp. Metastasis 21, 107–118 (2004).
JuanYin, J. et al. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin. Exp. Metastasis 26, 403–414 (2009).
Lorger, M., Krueger, J. S., O'Neal, M., Staflin, K. & Felding-Habermann, B. Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc. Natl Acad. Sci. USA 106, 10666–10671 (2009).
Hiratsuka, S. et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc. Natl Acad. Sci. USA 108, 302–307 (2011).
Bullitt, E. et al. Vessel tortuosity and brain tumor malignancy: a blinded study. Acad. Radiol. 12, 1232–1240 (2005).
Feigin, I., Allen, L. B., Lipkin, L. & Gross, S. W. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer 11, 264–277 (1958).
Yuan, F. et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564–4568 (1994).
Hiratsuka, S. et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin upregulation. Proc. Natl Acad. Sci. USA 108, 3725–3730 (2011).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Fukumura, D., Duda, D. G., Munn, L. L. & Jain, R. K. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 (2010).
Cranmer, L. D., Trevor, K. T., Bandlamuri, S. & Hersh, E. M. Rodent models of brain metastasis in melanoma. Melanoma Res. 15, 325–356 (2005).
Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R. S. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 68, 4500–4505 (2008).
Mathieu, A. et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-α, GST-μ, and GST-π. Cancer 101, 1908–1918 (2004).
Chen, E. I. et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 67, 1472–1486 (2007).
Monsky, W. L. et al. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin. Cancer Res. 8, 1008–1013 (2002).
Price, J. E. Metastasis from human breast cancer cell lines. Breast Cancer Res. Treat. 39, 93–102 (1996).
Price, J. E., Polyzos, A., Zhang, R. D. & Daniels, L. M. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 50, 717–721 (1990).
Rye, P. D. et al. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int. J. Cancer 68, 682–687 (1996).
Yoneda, T., Williams, P. J., Hiraga, T., Niewolna, M. & Nishimura, R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J. Bone Miner. Res. 16, 1486–1495 (2001).
Zhang, R. D., Fidler, I. J. & Price, J. E. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis 11, 204–215 (1991).
Fidler, I. J., Schackert, G., Zhang, R. D., Radinsky, R. & Fujimaki, T. The biology of melanoma brain metastasis. Cancer Metastasis Rev. 18, 387–400 (1999).
Palmieri, D. et al. HER-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 67, 4190–4198 (2007).
Gril, B. et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J. Natl Cancer Inst. 100, 1092–1103 (2008).
Izumi, Y., Xu, L., di Tomaso, E., Fukumura, D. & Jain, R. K. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416, 279–280 (2002).
Chung, E. et al. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS ONE 4, e8316 (2009).
Alterman, A. L. & Stackpole, C. W. B16 melanoma spontaneous brain metastasis: occurrence and development within leptomeninges blood vessels. Clin. Exp. Metastasis 7, 15–23 (1989).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Lockman, P. R. et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in mouse brain metastases of breast cancer. Clin. Cancer Res. 16, 5664–5678 (2010).
Weissleder, R. Scaling down imaging: molecular mapping of cancer in mice. Nat. Rev. Cancer 2, 11–18 (2002).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Anderson, S. A. & Frank, J. A. MRI of mouse models of neurological disorders. NMR Biomed. 20, 200–215 (2007).
Frank, J. A. et al. Methods for magnetically labeling stem and other cells for detection by in vivo magnetic resonance imaging. Cytotherapy 6, 621–625 (2004).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Song, H. T. et al. Quantitative T2* imaging of metastatic human breast cancer to brain in the nude rat at 3 T. NMR Biomed. 24, 325–334 (2011).
Song, H. T. et al. Rat model of metastatic breast cancer monitored by MRI at 3 tesla and bioluminescence imaging with histological correlation. J. Transl. Med. 7, 88 (2009).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Neuwelt, E. A. Mechanisms of disease: the blood-brain barrier. Neurosurgery 54, 131–140 (2004).
Deeken, J. F. & Loscher, W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin. Cancer Res. 13, 1663–1674 (2007).
Fukumura, D. et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 61, 6020–6024 (2001).
Hobbs, S. K. et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl Acad. Sci. USA 95, 4607–4612 (1998).
Monsky, W. L. et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 59, 4129–4135 (1999).
Zhang, R. D., Price, J. E., Fujimaki, T., Bucana, C. D. & Fidler, I. J. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am. J. Pathol. 141, 1115–1124 (1992).
Regina, A. et al. Multidrug resistance in brain tumors: roles of the blood-brain barrier. Cancer Metastasis Rev. 20, 13–25 (2001).
Patchell, R. A. et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280, 1485–1489 (1998).
de Boer, A. G. & Gaillard, P. J. Strategies to improve drug delivery across the blood-brain barrier. Clin. Pharmacokinet. 46, 553–576 (2007).
Lillis, A. P., Van Duyn, L. B., Murphy-Ullrich, J. E. & Strickland, D. K. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88, 887–918 (2008).
Pardridge, W. M. Re-engineering biopharmaceuticals for delivery to brain with molecular Trojan horses. Bioconjug. Chem. 19, 1327–1338 (2008).
Bu, G., Maksymovitch, E. A., Geuze, H. & Schwartz, A. L. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J. Biol. Chem. 269, 29874–29882 (1994).
Demeule, M. et al. High transcytosis of melanotransferrin (P97) across the blood-brain barrier. J. Neurochem. 83, 924–933 (2002).
Pan, W. et al. Efficient transfer of receptor-associated protein (RAP) across the blood-brain barrier. J. Cell Sci. 117, 5071–5078 (2004).
US National Library of Medicine. Clinicaltrials.gov [online], (2010).
US National Library of Medicine. Clinicaltrials.gov [online], (2010).
Regina, A. et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br. J. Pharmacol. 155, 185–197 (2008).
Thomas, F. C. et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 26, 2486–2494 (2009).
DeAngelis, L. M. et al. The combined use of radiation therapy and lonidamine in the treatment of brain metastases. J. Neurooncol. 7, 241–247 (1989).
Eyre, H. J. et al. Randomized trial of radiotherapy versus radiotherapy plus metronidazole for the treatment metastatic cancer to brain. A Southwest Oncology Group study. J. Neurooncol. 2, 325–330 (1984).
Komarnicky, L. T. et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int. J. Radiat. Oncol. Biol. Phys. 20, 53–58 (1991).
Mehta, M. P. et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 73, 1069–1076 (2009).
Phillips, T. L., Scott, C. B., Leibel, S. A., Rotman, M. & Weigensberg, I. J. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05 Int. J. Radiat. Oncol. Biol. Phys. 33, 339–348 (1995).
Suh, J. H. et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J. Clin. Oncol. 24, 106–114 (2006).
Neuhaus, T. et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br. J. Cancer 100, 291–297 (2009).
Baschnagel, A. et al. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol. Cancer Ther. 8, 1589–1595 (2009).
Palmieri, D. et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin. Cancer Res. 15, 6148–6157 (2009).
US National Library of Medicine. Clinicaltrials.gov [online], (2011).
US National Library of Medicine. Clinicaltrials.gov [online], (2010).
Besse, B. et al. Bevacizumab safety in patients with central nervous system metastases. Clin. Cancer Res. 16, 269–278 (2010).
Socinski, M. A. et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 27, 5255–5261 (2009).
Polikoff, J. et al. Safety of bevacizumab (Bv) therapy in combination with chemotherapy in subjects with non-small cell lung cancer (NSCLC) treated on ATLAS [abstract]. J. Clin. Oncol. 26 (15 Suppl.), a8079 (2008).
De Braganca, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J. Neurooncol. 100, 443–447 (2010).
Eichler, A. F. et al. A phase I study of cediranib plus whole-brain radiation therapy in patients with brain metastases from non-small cell lung cancer [abstract]. J. Clin. Oncol. 28 (15 Suppl.), TPS177 (2010).
Ceresoli, G. L. et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann. Oncol. 15, 1042–1047 (2004).
Wu, C. et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 57, 359–364 (2007).
Fekrazad, M. H., Ravindranathan, M. & Jones, D. V. Jr. Response of intracranial metastases to erlotinib therapy. J. Clin. Oncol. 25, 5024–5026 (2007).
Lai, C. S., Boshoff, C., Falzon, M. & Lee, S. M. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax 61, 91 (2006).
Popat, S. et al. Recurrent responses to non-small cell lung cancer brain metastases with erlotinib. Lung Cancer 56, 135–137 (2007).
Kim, J. E. et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 65, 351–354 (2009).
Paz-Ares, L. et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 24, 1428–1434 (2006).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Leyland-Jones, B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J. Clin. Oncol. 27, 5278–5286 (2009).
Lin, N. U. & Winer, E. P. Brain metastases: the HER2 paradigm. Clin. Cancer Res. 13, 1648–1655 (2007).
Zhang, L., Sullivan, P. S., Goodman, J. C., Gunaratne, P. H. & Marchetti, D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 71, 645–654 (2011).
Lin, N. U. et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 26, 1993–1999 (2008).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15, 1452–1459 (2009).
Cameron, D. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112, 533–543 (2008).
US National Library of Medicine. Clinicaltrials.gov [online], (2011).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
Long, G. V. et al. Phase 1/2 study of GSK2118436, a selective inhibitor of V600 mutant (MUT) BRAF kinase; evidence of activity in melanoma brain metastases (METS) [abstract]. Ann. Oncol. 21, viii12 (2010).
Felding-Habermann, B. et al. Integrin activation controls metastasis in human breast cancer. Proc. Natl Acad. Sci. USA 98, 1853–1858 (2001).
Palmieri, D. et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 7, 1438–1445 (2009).
Ray, P. S. et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 70, 3870–3876 (2010).
Nguyen, D. X. et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009).
Grinberg-Rashi, H. et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin. Cancer Res. 15, 1755–1761 (2009).
Xie, T. X. et al. Activation of Stat3 in human melanoma promotes brain metastasis. Cancer Res. 66, 3188–3196 (2006).
Nam, D. H. et al. Activation of notch signaling in a xenograft model of brain metastasis. Clin. Cancer Res. 14, 4059–4066 (2008).
Siegal, T. et al. Alteration of blood-brain-CSF barrier in experimental meningeal carcinomatosis. A morphologic and adriamycin-penetration study. J. Neurooncol. 4, 233–242 (1987).
Schabet, M. & Herrlinger, U. Animal models of leptomeningeal metastasis. J. Neurooncol. 38, 199–205 (1998).
Schackert, G., Price, J. E., Bucana, C. D. & Fidler, I. J. Unique patterns of brain metastasis produced by different human carcinomas in athymic nude mice. Int. J. Cancer 44, 892–897 (1989).
Broder, H., Anderson, A., Kremen, T. J., Odesa, S. K. & Liau, L. M. MART-1 adenovirus-transduced dendritic cell immunization in a murine model of metastatic central nervous system tumor. J. Neurooncol. 64, 21–30 (2003).
Schackert, G. & Fidler, I. J. Development of in vivo models for studies of brain metastasis. Int. J. Cancer 41, 589–594 (1988).
Schackert, G. & Fidler, I. J. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 48, 3478–3484 (1988).
Yang, M. et al. Genetically fluorescent melanoma bone and organ metastasis models. Clin. Cancer Res. 5, 3549–3559 (1999).
Muldoon, L. L. et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J. Clin. Oncol. 25, 2295–2305 (2007).
Bellavance, M. A., Blanchette, M. & Fortin, D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 10, 166–177 (2008).
Gregor, A. et al. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J. Neurooncol. 44, 137–145 (1999).
Matsukado, K. et al. Enhanced tumor uptake of carboplatin and survival in glioma-bearing rats by intracarotid infusion of bradykinin analog, RMP-7. Neurosurgery 39, 125–133 (1996).
Prados, M. D. et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 5, 96–103 (2003).
Jones, A. R. & Shusta, E. V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 24, 1759–1771 (2007).
Joo, K. M. et al. Oral paclitaxel chemotherapy for brain tumors: ideal combination treatment of paclitaxel and p-glycoprotein inhibitor. Oncol. Rep. 19, 17–23 (2008).
Joo, K. M. et al. Response of brain specific microenvironment to p-glycoprotein inhibitor: an important factor determining therapeutic effect of p-glycoprotein inhibitor on brain metastatic tumors. Int. J. Oncol. 33, 705–712 (2008).
Bauer, B., Hartz, A. M., Fricker, G. & Miller, D. S. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp. Biol. Med. 230, 118–127 (2005).
Acknowledgements
We thank J. Engelman, S. Goel, L. Xu, I. J. Fidler, R. S. Kerbel, W. Cruz-Munoz and S. Mohla for their helpful comments on the manuscript. This work was supported by the National Institutes of Health grants P01-CA080124 (R. K. Jain and D. Fukumura), R01-CA085140, R01-CA115767 and R01-CA126642 (R. K. Jain), R01-CA096915 (D. Fukumura), R21-CA135605 and U01-CA062490 (A. F. Eichler), a Federal Share Income Grant (R. K. Jain and D. Fukumura), T32-CA073479 (D. P. Kodak) and Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (R. K. Jain). E. Chung was supported by a Tosteson postdoctoral fellowship award from the Massachusetts Biomedical Research Corporation. E. Chung also acknowledges support from his current institute: Gwangju Institute of Science and Technology, Gwangju, Republic of Korea.
Author information
Authors and Affiliations
Contributions
A. F. Eichler and R. K. Jain contributed equally to the preparation of this manuscript. All authors contributed to researching data for the article, discussions of content, writing of the manuscript, and to reviewing and editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
R. K. Jain declares receiving consulting fees from Astellas, AstraZeneca, Dyax, Enlight Biosciences, Genzyme, Millenium, Noxxon and SynDevRx; lecture fees from MPM Capital; grant support from Dyax, AstraZeneca/MedImmune and Roche; and owning equity in Enlight Biosciences and SynDevRx. Other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Eichler, A., Chung, E., Kodack, D. et al. The biology of brain metastases—translation to new therapies. Nat Rev Clin Oncol 8, 344–356 (2011). https://doi.org/10.1038/nrclinonc.2011.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2011.58
This article is cited by
-
The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: when the dialogue replaces the monologue
Cell & Bioscience (2023)
-
Albumin-binding photosensitizer capable of targeting glioma via the SPARC pathway
Biomaterials Research (2023)
-
Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2
Nature Cancer (2023)
-
The blood–brain barrier: structure, regulation, and drug delivery
Signal Transduction and Targeted Therapy (2023)
-
Präoperative Bestrahlung von Hirnmetastasen
Die Onkologie (2023)