Key Points
-
Paroxysmal atrial fibrillation (pAF) has discrete clinical features from persistent forms, including greater relative pathophysiological importance of the pulmonary vein sleeves and greater susceptibility to both medical and ablation therapy
-
Evidence indicates that both focal ectopic activity and re-entry can have a role in pAF, and that the pulmonary veins have structural and electrophysiological features that favour both mechanisms
-
The mechanisms underlying pAF are likely to vary between patients, depending on factors such as genetic background, cardiovascular risk factors, and concomitant heart disease
-
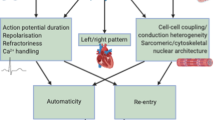
Ca2+-dependent triggered activity seems to underlie atrial ectopy in pAF, and has complex underlying molecular mechanisms that increase both cellular Ca2+ load and the leakiness of the sarcoplasmic reticulum Ca2+-release channel (ryanodine receptor)
-
Molecular mechanisms promoting re-entrant activity in patients with pAF include ionic properties (such as larger left atrial inward-rectifier background current) and structural properties (such as atrial fibrosis)
-
Fairly little attention has been paid in the literature to the specific mechanistic basis of pAF; more work is needed to provide insights with translational potential
Abstract
Atrial fibrillation (AF) is an extremely prevalent arrhythmia that presents a wide range of therapeutic challenges. AF usually begins in a self-terminating paroxysmal form (pAF). With time, the AF pattern often evolves to become persistent (nonterminating within 7 days). Important differences exist between pAF and persistent AF in terms of clinical features, in particular the responsiveness to antiarrhythmic drugs and ablation therapy. AF mechanisms have been extensively reviewed, but few or no Reviews focus specifically on the pathophysiology of pAF. Accordingly, in this Review, we examine the available data on the electrophysiological basis for pAF occurrence and maintenance, as well as the molecular mechanisms forming the underlying substrate. We first consider the mechanistic insights that have been obtained from clinical studies in the electrophysiology laboratory, noninvasive observations, and genetic studies. We then discuss the information about underlying molecular mechanisms that has been obtained from experimental studies on animal models and patient samples. Finally, we discuss the data available from animal models with spontaneous AF presentation, their relationship to clinical findings, and their relevance to understanding the mechanisms underlying pAF. Our analysis then turns to potential factors governing cases of progression from pAF to persistent AF and the clinical implications of the basic mechanisms we review. We conclude by identifying and discussing questions that we consider particularly important to address through future research in this area.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Heijman, J., Voigt, N. & Dobrev, D. New directions in antiarrhythmic drug therapy for atrial fibrillation. Future Cardiol. 9, 71–88 (2013).
Dobrev, D. & Nattel, S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet 375, 1212–1223 (2010).
Calkins, H. et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14, 528–606 (2012).
Wakili, R., Voigt, N., Kääb, S., Dobrev, D. & Nattel, S. Recent advances in the molecular pathophysiology of atrial fibrillation. J. Clin. Invest. 121, 2955–2968 (2011).
Heijman, J., Voigt, N., Nattel, S. & Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 114, 1483–1499 (2014).
Schotten, U., Dobrev, D., Platonov, P. G., Kottkamp, H. & Hindricks, G. Current controversies in determining the main mechanisms of atrial fibrillation. J. Intern. Med. 279, 428–438 (2016).
Nattel, S., Burstein, B. & Dobrev, D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ. Arrhythm. Electrophysiol. 1, 62–73 (2008).
Nattel, S. et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur. Heart J. 35, 1448–1456 (2014).
Lim, H. S. et al. Persistent atrial fibrillation from the onset: a specific subgroup of patients with biatrial substrate involvement and poorer clinical outcome. JACC Clin. Electrophysiol. 2, 129–139 (2016).
Oral, H. et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 105, 1077–1081 (2002).
Eijsbouts, S. et al. Serial cardioversion by class IC Drugs during 4 months of persistent atrial fibrillation in the goat. J. Cardiovasc. Electrophysiol. 17, 648–654 (2006).
Haissaguerre, M. et al. Driver domains in persistent atrial fibrillation. Circulation 130, 530–538 (2014).
Jaïs, P. et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 95, 572–576 (1997).
Haïssaguerre, M. et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 339, 659–666 (1998).
Jaïs, P. et al. Ablation therapy for atrial fibrillation (AF): past, present and future. Cardiovasc. Res. 54, 337–346 (2002).
Macle, L. et al. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet 386, 672–679 (2015).
Drewitz, I. et al. Persistent, isolated pulmonary vein re-entry: inducibility, entrainment, and overdrive termination of a sustained tachycardia within an isolated pulmonary vein. Europace 10, 261–264 (2008).
Atienza, F. et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation 114, 2434–2442 (2006).
Nattel, S. Adenosine and atrial arrhythmias: exploring electrophysiological mechanisms in vivo. Pacing Clin. Electrophysiol. 35, 553–555 (2012).
Teh, A. W. et al. Electroanatomic properties of the pulmonary veins: slowed conduction, low voltage and altered refractoriness in AF patients. J. Cardiovasc. Electrophysiol. 22, 1083–1091 (2011).
Teh, A. W. et al. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J. Cardiovasc. Electrophysiol. 23, 232–238 (2012).
Stiles, M. K. et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the 'second factor'. J. Am. Coll. Cardiol. 53, 1182–1191 (2009).
Cozma, D. et al. Mechanism of atrial fibrillation: decremental conduction, fragmentation, and ectopic activity in patients with drug resistance paroxysmal atrial fibrillation and structurally normal heart. Pacing Clin. Electrophysiol. 28, S115–S119 (2005).
Yamabe, H., Kanazawa, H., Itoh, M., Kaneko, S. & Ogawa, H. Difference in the maintenance mechanism of atrial fibrillation perpetuated after pulmonary vein isolation between paroxysmal and persistent atrial fibrillation: effects of subsequent stepwise ablation. Int. J. Cardiol. 210, 109–118 (2016).
Vincenti, A., Brambilla, R., Fumagalli, M. G., Merola, R. & Pedretti, S. Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace 8, 204–210 (2006).
Bettoni, M. & Zimmermann, M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 105, 2753–2759 (2002).
Rosso, R. et al. Vagal paroxysmal atrial fibrillation: prevalence and ablation outcome in patients without structural heart disease. J. Cardiovasc. Electrophysiol. 21, 489–493 (2010).
Waktare, J. E. et al. The role of atrial ectopics in initiating paroxysmal atrial fibrillation. Eur. Heart J. 22, 333–339 (2001).
Hattori, T. et al. A novel gain-of-function KCNJ2 mutation associated with short-QT syndrome impairs inward rectification of Kir2.1 currents. Cardiovasc. Res. 93, 666–673 (2012).
Olesen, M. S. et al. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Med. Genet. 13, 24 (2012).
Deo, M. et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc. Natl Acad. Sci. USA 110, 4291–4296 (2013).
Hong, K., Bjerregaard, P., Gussak, I. & Brugada, R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J. Cardiovasc. Electrophysiol. 16, 394–396 (2005).
Zhang, X. et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell 135, 1017–1027 (2008).
Kazemian, P., Gollob, M. H., Pantano, A. & Oudit, G. Y. A novel mutation in the RYR2 gene leading to catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation: dose-dependent arrhythmia-event suppression by β-blocker therapy. Can. J. Cardiol. 27, 870.e7–10 (2011).
Beavers, D. L. et al. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J. Am. Coll. Cardiol. 62, 2010–2019 (2013).
Chiang, D. Y. et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ. Arrhythm. Electrophysiol. 7, 1214–1222 (2014).
Ellinor, P. T., Petrov-Kondratov, V. I., Zakharova, E., Nam, E. G. & MacRae, C. A. Potassium channel gene mutations rarely cause atrial fibrillation. BMC Med. Genet. 7, 70 (2006).
Nattel, S. Paroxysmal atrial fibrillation and pulmonary veins: relationships between clinical forms and automatic versus re-entrant mechanisms. Can. J. Cardiol. 29, 1147–1149 (2013).
Lindberg, S., Hansen, S. & Nielsen, T. Spontaneous conversion of first onset atrial fibrillation. Intern. Med. J. 42, 1195–1199 (2012).
Verheule, S. et al. Tissue structure and connexin expression of canine pulmonary veins. Cardiovasc. Res. 55, 727–738 (2002).
Watanabe, E. I. et al. Modulation of pacemaker activity of sinoatrial node cells by electrical load imposed by an atrial cell model. Am. J. Physiol. 269, H1735–H1742 (1995).
Ehrlich, J. R. et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J. Physiol. 551, 801–813 (2003).
Hocini, M. et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 105, 2442–2448 (2002).
Ozgen, N. et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc. Res. 75, 758–769 (2007).
Qi, X.-Y. et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 129, 430–440 (2014).
Pandit, S. V. et al. Ionic determinants of functional reentry in a 2D model of human atrial cells during simulated chronic atrial fibrillation. Biophys. J. 88, 3806–3821 (2005).
Voigt, N. et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 129, 145–156 (2014).
Zhao, F. et al. Calreticulin overexpression correlates with integrin-α5 and transforming growth factor-β1 expression in the atria of patients with rheumatic valvular disease and atrial fibrillation. Int. J. Cardiol. 168, 2177–2185 (2013).
Voigt, N. et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125, 2059–2070 (2012).
Chiang, D. Y. et al. Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients. J. Am. Coll. Cardiol. 65, 163–173 (2015).
Brundel, B. J. et al. Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc. Res. 42, 443–454 (1999).
Smith, S. A. et al. Dysfunction in the βII spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation 131, 695–708 (2015).
Smith, S. et al. Dysfunction of the βII spectrin-based pathway in human heart failure. Am. J. Physiol. Heart Circ. Physiol. 310, H1583–H1591 (2016).
Cunha, S. R. et al. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 124, 1212–1222 (2011).
Schmidt, C. et al. Upregulation of K2P3.1 K+ current causes action potential shortening in patients with chronic atrial fibrillation. Circulation 132, 82–92 (2015).
Ford, J. et al. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm 13, 555–564 (2016).
Voigt, N. et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ. Arrhythm. Electrophysiol. 3, 472–480 (2010).
Glasscock, E. et al. Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Bas. Res. Cardiol. 110, 505 (2015).
Zhou, X. et al. Increased trafficking of Ca2+-activated K+ channels to plasma membrane modulates action potential duration in human paroxysmal atrial fibrillation. Eur. Heart J. 35 (Suppl. 1), 183 [abstract 1041] (2014).
Brundel, B. J. et al. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J. Am. Coll. Cardiol. 37, 926–932 (2001).
Brundel, B. J. et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation 103, 684–690 (2001).
Voigt, N. et al. Impaired Na+-dependent regulation of acetylcholine-activated inward-rectifier K+ current modulates action potential rate dependence in patients with chronic atrial fibrillation. J. Mol. Cell. Cardiol. 61, 142–152 (2013).
Chiang, D. Y. et al. Identification of microRNA-mRNA dysregulations in paroxysmal atrial fibrillation. Int. J. Cardiol. 184, 190–197 (2015).
Harada, M. et al. Atrial fibrillation activates AMP-dependent protein kinase and its regulation of cellular calcium handling: potential role in metabolic adaptation and prevention of progression. J. Am. Coll. Cardiol. 66, 47–58 (2015).
Li, N. et al. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 129, 1276–1285 (2014).
Gemel, J. et al. Connexin40 abnormalities and atrial fibrillation in the human heart. J. Mol. Cell. Cardiol. 76, 159–168 (2014).
Brundel, B. J. J. M. et al. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc. Res. 54, 380–389 (2002).
Ke, L. et al. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J. Mol. Cell. Cardiol. 45, 685–693 (2008).
Zhang, D. et al. Activation of histone deacetylase-6 induces contractile dysfunction through derailment of α-tubulin proteostasis in experimental and human atrial fibrillation. Circulation 129, 346–358 (2014).
Carnes, C. A. et al. Atrial glutathione content, calcium current, and contractility. J. Biol. Chem. 282, 28063–28073 (2007).
Brundel, B. J. J. M. et al. Heat shock protein upregulation protects against pacing-induced myolysis in HL-1 atrial myocytes and in human atrial fibrillation. J. Mol. Cell. Cardiol. 41, 555–562 (2006).
Goette, A. et al. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 101, 2678–2681 (2000).
Xiao, H., Lei, H., Qin, S., Ma, K. & Wang, X. TGF-β1 expression and atrial myocardium fibrosis increase in atrial fibrillation secondary to rheumatic heart disease. Clin. Cardiol. 33, 149–156 (2010).
Brundel, B. J. et al. Endothelin system in human persistent and paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 12, 737–742 (2001).
Goette, A. et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 35, 1669–1677 (2000).
Nakano, Y. et al. Matrix metalloproteinase-9 contributes to human atrial remodeling during atrial fibrillation. J. Am. Coll. Cardiol. 43, 818–825 (2004).
Cao, H. et al. Natriuretic peptides and right atrial fibrosis in patients with paroxysmal versus persistent atrial fibrillation. Peptides 31, 1531–1539 (2010).
Platonov, P. G., Mitrofanova, L. B., Orshanskaya, V. & Ho, S. Y. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J. Am. Coll. Cardiol. 58, 2225–2232 (2011).
Haemers, P. et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehv625 (2015).
Nishida, K., Michael, G., Dobrev, D. & Nattel, S. Animal models for atrial fibrillation: clinical insights and scientific opportunities. Europace 12, 160–172 (2010).
Riley, G., Syeda, F., Kirchhof, P. & Fabritz, L. An introduction to murine models of atrial fibrillation. Front. Physiol. 3, 296 (2012).
Nattel, S., Shiroshita-Takeshita, A., Brundel, B. J. J. M. & Rivard, L. Mechanisms of atrial fibrillation: lessons from animal models. Prog. Cardiovasc. Dis. 48, 9–28 (2005).
Finet, J. E., Rosenbaum, D. S. & Donahue, J. K. Information learned from animal models of atrial fibrillation. Cardiol. Clin. 27, 45–54 (2009).
Martins, R. P. et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 129, 1472–1482 (2014).
Müller, F. U. et al. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J. Biol. Chem. 280, 6906–6914 (2005).
Kirchhof, P. et al. Overexpression of cAMP-response element modulator causes abnormal growth and development of the atrial myocardium resulting in a substrate for sustained atrial fibrillation in mice. Int. J. Cardiol. 166, 366–374 (2013).
Glukhov, A. V. et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur. Heart J. 36, 686–697 (2015).
Levin, M. D. et al. Melanocyte-like cells in the heart and pulmonary veins contribute to atrial arrhythmia triggers. J. Clin. Invest. 119, 3420–3436 (2009).
Wan, E. et al. Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J. Clin. Invest. 126, 112–122 (2016).
Temple, J. et al. Atrial fibrillation in KCNE1-null mice. Circ. Res. 97, 62–69 (2005).
Li, J., McLerie, M. & Lopatin, A. N. Transgenic upregulation of IK1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am. J. Physiol. Heart Circ. Physiol. 287, H2790–2802 (2004).
Hirose, M. et al. Diacylglycerol kinase ζ inhibits Gαq-induced atrial remodeling in transgenic mice. Heart Rhythm 6, 78–84 (2009).
Hong, C.-S. et al. Overexpression of junctate induces cardiac hypertrophy and arrhythmia via altered calcium handling. J. Mol. Cell. Cardiol. 44, 672–682 (2008).
Hong, C.-S. et al. Cardiac remodeling and atrial fibrillation in transgenic mice overexpressing junctin. FASEB J. 16, 1310–1312 (2002).
Choi, E.-K. et al. Triggered firing and atrial fibrillation in transgenic mice with selective atrial fibrosis induced by overexpression of TGF-β1. Circ. J. 76, 1354–1362 (2012).
Ozcan, C., Battaglia, E., Young, R. & Suzuki, G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J. Am. Heart Assoc. 4, e001733 (2015).
Xiao, H. D. et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am. J. Pathol. 165, 1019–1032 (2004).
Arad, M. et al. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation 107, 2850–2856 (2003).
Watanabe, H. et al. Striking in vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation 124, 1001–1011 (2011).
Pretorius, L. et al. Reduced phosphoinositide 3-kinase (p110α) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 175, 998–1009 (2009).
Chen, H.-H. et al. Transcription enhancer factor-1-related factor-transgenic mice develop cardiac conduction defects associated with altered connexin phosphorylation. Circulation 110, 2980–2987 (2004).
Sawaya, S. E. et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am. J. Physiol. Heart Circ. Physiol. 292, H1561–1567 (2007).
Adam, O. et al. Role of Rac1 GTPase activation in atrial fibrillation. J. Am. Coll. Cardiol. 50, 359–367 (2007).
Scridon, A. et al. Unprovoked atrial tachyarrhythmias in aging spontaneously hypertensive rats: the role of the autonomic nervous system. Am. J. Physiol. Heart Circ. Physiol. 303, H386–H392 (2012).
Scridon, A. et al. Long-standing arterial hypertension is associated with Pitx2 down-regulation in a rat model of spontaneous atrial tachyarrhythmias. Europace 17, 160–165 (2015).
Nishida, K. et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation 123, 137–146 (2011).
Yeh, Y.-H. et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ. Arrhythm. Electrophysiol. 1, 93–102 (2008).
Burstein, B. et al. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ. Res. 105, 1213–1222 (2009).
Wakili, R. et al. Temporal evolution of atrial remodeling and atrial arrhythmogenesis during tachycardiomyopathic heart failure development. Circulation 122, A17762 (2010).
Tan, A. Y. et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation 118, 916–925 (2008).
Choi, E.-K. et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation 121, 2615–2623 (2010).
Sharifov, O. F. et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J. Am. Coll. Cardiol. 43, 483–490 (2004).
Carneiro, J. S. et al. The selective cardiac late sodium current inhibitor GS-458967 suppresses autonomically triggered atrial fibrillation in an intact porcine model. J. Cardiovasc. Electrophysiol. 26, 1364–1369 (2015).
Zicha, S., Fernández-Velasco, M., Lonardo, G., L'Heureux, N. & Nattel, S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc. Res. 66, 472–481 (2005).
Qi, X.-Y. et al. Fibroblast inward-rectifier potassium current upregulation in profibrillatory atrial remodeling. Circ. Res. 116, 836–845 (2015).
Aguilar, M., Qi, X. Y., Huang, H., Comtois, P. & Nattel, S. Fibroblast electrical remodeling in heart failure and potential effects on atrial fibrillation. Biophys. J. 107, 2444–2455 (2014).
Harada, M. et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation 126, 2051–2064 (2012).
Jost, N. et al. Ionic mechanisms limiting cardiac repolarization reserve in humans compared to dogs. J. Physiol. 591, 4189–4206 (2013).
De Vos, C. B. et al. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm-control therapy. Am. Heart J. 163, 887–893 (2012).
de Vos, C. B. et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 55, 725–731 (2010).
Wijffels, M. C., Kirchhof, C. J., Dorland, R. & Allessie, M. A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92, 1954–1968 (1995).
Yue, L. et al. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 81, 512–525 (1997).
Qi, X. Y. et al. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ. Res. 103, 845–854 (2008).
Luo, X. et al. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Invest. 123, 1939–1951 (2013).
Harada, M. et al. MicroRNA regulation and cardiac calcium signaling: role in cardiac disease and therapeutic potential. Circ. Res. 114, 689–705 (2014).
Ausma, J. et al. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 96, 3157–3163 (1997).
Kirubakaran, S. et al. Fractionation of electrograms is caused by colocalized conduction block and connexin disorganization in the absence of fibrosis as AF becomes persistent in the goat model. Heart Rhythm 12, 397–408 (2015).
Verheule, S. et al. Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ. Arrhythm. Electrophysiol. 6, 202–211 (2013).
Burstein, B., Qi, X.-Y., Yeh, Y.-H., Calderone, A. & Nattel, S. Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: a novel consideration in atrial remodeling. Cardiovasc. Res. 76, 442–452 (2007).
Garratt, C. J. et al. Repetitive electrical remodeling by paroxysms of atrial fibrillation in the goat: no cumulative effect on inducibility or stability of atrial fibrillation. J. Cardiovasc. Electrophysiol. 10, 1101–1108 (1999).
Todd, D. M. et al. Repetitive 4-week periods of atrial electrical remodeling promote stability of atrial fibrillation: time course of a second factor involved in the self-perpetuation of atrial fibrillation. Circulation 109, 1434–1439 (2004).
Wu, C.-T. et al. Repeated paroxysmal atrial fibrillation episodes remodel ionic currents and promote atrial fibrillation in dogs. Circulation 128, [abstract 13765] (2013).
Kirchhof, P. et al. Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 15, 1540–1556 (2013).
Donahue, J. K. Biological therapies for atrial fibrillation: ready for prime time? J. Cardiovasc. Pharmacol. 67, 19–25 (2016).
Nishida, K., Datino, T., Macle, L. & Nattel, S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J. Am. Coll. Cardiol. 64, 823–831 (2014).
Datino, T. et al. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation 121, 963–972 (2010).
Chen, P.-S., Chen, L. S., Fishbein, M. C., Lin, S.-F. & Nattel, S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ. Res. 114, 1500–1515 (2014).
Podd, S. J., Freemantle, N., Furniss, S. S. & Sulke, N. First clinical trial of specific IKACh blocker shows no reduction in atrial fibrillation burden in patients with paroxysmal atrial fibrillation: pacemaker assessment of BMS 914392 in patients with paroxysmal atrial fibrillation. Europace 18, 340–346 (2016).
Heijman, J. et al. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc. Res. 109, 467–479 (2016).
Pathak, R. K., Mahajan, R., Lau, D. H. & Sanders, P. The implications of obesity for cardiac arrhythmia mechanisms and management. Can. J. Cardiol. 31, 203–210 (2015).
Gal, P. & Marrouche, N. F. Magnetic resonance imaging of atrial fibrosis: redefining atrial fibrillation to a syndrome. Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehv514 (2015).
Rudy, Y. Noninvasive electrocardiographic imaging of arrhythmogenic substrates in humans. Circ. Res. 112, 863–874 (2013).
Watson, T., Shantsila, E. & Lip, G. Y. H. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 373, 155–166 (2009).
Nattel, S. New ideas about atrial fibrillation 50 years on. Nature 415, 219–226 (2002).
Mancarella, S. et al. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the α1D L-type Ca2+ channel KO mouse. Am. J. Physiol. Heart Circ. Physiol. 295, H2017–H2024 (2008).
Acknowledgements
The authors were supported by grants from the Canadian Institutes of Health Research (S.N.), Quebec Heart and Stroke Foundation (S.N.), the European-North American Atrial Fibrillation Research Alliance (07CVD03) grant from Fondation Leducq (S.N., D.D.), the European Network for Translational Research in Atrial Fibrillation (EUTRAF, No. 261057; D.D.), the DZHK (German Center for Cardiovascular Research; D.D.), and the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH RO1HL131517; D.D.).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed/edited it before submission.
Corresponding author
Ethics declarations
Competing interests
D.D. declares that he has received consultancy fees and research grants from OMEICOS Therapeutics GmbH and Xention. S.N. declares no competing interests.
Rights and permissions
About this article
Cite this article
Nattel, S., Dobrev, D. Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation. Nat Rev Cardiol 13, 575–590 (2016). https://doi.org/10.1038/nrcardio.2016.118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.118
This article is cited by
-
Intersection of sepsis, atrial fibrillation, and severe obesity: a population-based analysis in the United States
International Journal of Obesity (2024)
-
Pathophysiology and clinical relevance of atrial myopathy
Basic Research in Cardiology (2024)
-
Obesity and atrial fibrillation: a narrative review from arrhythmogenic mechanisms to clinical significance
Cardiovascular Diabetology (2023)
-
Risk factors associated with left atrial appendage thrombosis in patients with non-valvular atrial fibrillation by transesophageal echocardiography
The International Journal of Cardiovascular Imaging (2023)
-
Mouse models of spontaneous atrial fibrillation
Mammalian Genome (2023)