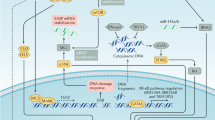
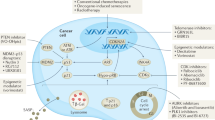
Abstract
Recently, it has been shown that oncogene-induced senescence (OIS) occurs during the early stages of tumorigenesis. Senescent tumour cells are abundant within premalignant neoplastic lesions, whereas they are scarce in malignant tumours. This association of senescence with the premalignant stages of tumour progression opens the possibility of using senescence markers as diagnostic and prognostic tools. Moreover, some chemotherapeutic protocols induce senescence in tumour cells and, consequently, senescence markers could help to monitor treatment response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Serrano, M. & Blasco, M. A. Putting the stress on senescence. Curr. Opin. Cell Biol. 13, 748–753 (2001).
Shay, J. W. & Roninson, I. B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 23, 2919–2933 (2004).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Guerra, C. et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4, 111–120 (2003).
Tuveson, D. A. et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Lo, T. L. et al. Sprouty and cancer: the first terms report. Cancer Lett. 6 Feb 2006 [epub ahead of print].
Furukawa, T., Sunamura, M., Motoi, F., Matsuno, S. & Horii, A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am. J. Pathol. 162, 1807–1815 (2003).
Hoornaert, I., Marynen, P., Goris, J., Sciot, R. & Baens, M. MAPK phosphatase DUSP16/MKP-7, a candidate tumor suppressor for chromosome region 12p12–13, reduces BCR-ABL-induced transformation. Oncogene 22, 7728–7736 (2003).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Kurz, D. J., Decary, S., Hong, Y. & Erusalimsky, J. D. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113, 3613–3622 (2000).
Severino, J., Allen, R. G., Balin, S., Balin, A. & Cristofalo, V. J. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp. Cell Res. 257, 162–171 (2000).
Yang, N. C. & Hu, M. L. A fluorimetric method using fluorescein di-beta-D-galactopyranoside for quantifying the senescence-associated beta-galactosidase activity in human foreskin fibroblast Hs68 cells. Anal. Biochem. 325, 337–343 (2004).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Hollstein, M., Sidransky, D., Vogelstein, B. & Harris, C. C. p53 mutations in human cancers. Science 253, 49–53 (1991).
Ruas, M. & Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim Biophys. Acta. 1378, F115–F177 (1998).
Sharpless, N. E. & DePinho, R. A. The INK4A/ARF locus and its two gene products. Curr. Opin. Genet. Dev. 9, 22–30 (1999).
Lazzerini Denchi, E., Attwooll, C., Pasini, D. & Helin, K. Deregulated E2F activity induces hyperplasia and senescence-like features in the mouse pituitary gland. Mol. Cell. Biol. 25, 2660–2672 (2005).
Shapiro, G. I. et al. Reciprocal Rb inactivation and p16INK4 expression in primary lung cancers and cell lines. Cancer Res. 55, 505–509 (1995).
Masumoto, N. et al. P16 overexpression and human papillomavirus infection in small cell carcinoma of the uterine cervix. Hum. Pathol. 34, 778–783 (2003).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Zhang, R. et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
Malumbres, M. et al. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15(INK4b). Mol. Cell. Biol. 20, 2915–2925 (2000).
Nagane, M., Huang, H. J. & Cavenee, W. K. The potential of TRAIL for cancer chemotherapy. Apoptosis 6, 191–197 (2001).
Giatromanolaki, A. et al. DEC1 (STRA13) protein expression relates to hypoxia- inducible factor 1-alpha and carbonic anhydrase-9 overexpression in non-small cell lung cancer. J. Pathol. 200, 222–228 (2003).
Liu, X., Yue, P., Khuri, F. R. & Sun, S. Y. Decoy receptor 2 (DcR2) is a p53 target gene and regulates chemosensitivity. Cancer. Res. 65, 9169–9175 (2005).
Yamada, K. & Miyamoto, K. Basic helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their gene expressions are regulated by multiple extracellular stimuli. Front. Biosci. 10, 3151–3171 (2005).
Barradas, M. et al. Identification of a candidate tumor-suppressor gene specifically activated during Ras-induced senescence. Exp. Cell Res. 273, 127–137 (2002).
Hama, T. et al. Identification and molecular cloning of a novel brain-specific receptor protein that binds to brain injury-derived neurotrophic peptide. Possible role for neuronal survival. J. Biol. Chem. 276, 31929–31935 (2001).
Brunk, U. T. & Terman, A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 (2002).
Gerland, L. M. et al. Association of increased autophagic inclusions labeled for beta-galactosidase with fibroblastic aging. Exp. Gerontol. 38, 887–895 (2003).
Mu, X. C. & Higgins, P. J. Differential growth state-dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J. Cell. Physiol. 165, 647–657 (1995).
Mason, D. X., Jackson, T. J. & Lin, A. W. Molecular signature of oncogenic ras-induced senescence. Oncogene 23, 9238–9246 (2004).
Chang, B. D. et al. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc. Natl Acad. Sci. USA 99, 389–394 (2002).
Schmitt, C. A. Senescence, apoptosis and therapy – cutting the lifelines of cancer. Nature Rev. Cancer. 3, 286–295 (2003).
Rebbaa, A., Zheng, X., Chou, P. M. & Mirkin, B. L. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene 22, 2805–2811 (2003).
Zheng, X., Chou, P. M., Mirkin, B. L. & Rebbaa, A. Senescence-initiated reversal of drug resistance: specific role of cathepsin L. Cancer Res. 64, 1773–1780 (2004).
te Poele, R. H., Okorokov, A. L., Jardine, L., Cummings, J. & Joel, S. P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 62, 1876–1883 (2002).
Roberson, R. S., Kussick, S. J., Vallieres, E., Chen, S. Y. & Wu, D. Y. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 65, 2795–2803 (2005).
Sedelnikova, O. A. et al. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nature Cell Biol. 6, 168–170 (2004).
Shelton, D. N., Chang, E., Whittier, P. S., Choi, D. & Funk, W. D. Microarray analysis of replicative senescence. Curr. Biol. 9, 939–645 (1999).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
Alcorta, D. A. et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA 93, 13742–13747 (1996).
Afshari, C. A. et al. Investigation of the role of G1/S cell cycle mediators in cellular senescence. Exp. Cell Res. 209, 231–237 (1993).
Kamijo, T. et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Tahara, H., Sato, E., Noda, A. & Ide, T. Increase in expression level of p21sdi1/cip1/waf1 with increasing division age in both normal and SV40-transformed human fibroblasts. Oncogene 10, 835–840 (1995).
Acknowledgements
Research at the laboratory of M.S. is funded by the Centro Nacional de Investigaciones Oncológicas (CNIO), the Spanish Ministry of Education and Science, and the European Union (INTACT and PROTEOMAGE projects).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Collado, M., Serrano, M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer 6, 472–476 (2006). https://doi.org/10.1038/nrc1884
Issue Date:
DOI: https://doi.org/10.1038/nrc1884
This article is cited by
-
Protooncogene MYC drives human melanocyte melanogenesis and senescence
Cancer Gene Therapy (2022)
-
USP5-Beclin 1 axis overrides p53-dependent senescence and drives Kras-induced tumorigenicity
Nature Communications (2022)
-
Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection
Pharmaceutical Medicine (2022)
-
Contribution of senescent and reactive astrocytes on central nervous system inflammaging
Biogerontology (2022)
-
The oncogenic E3 ligase TRIP12 suppresses epithelial–mesenchymal transition (EMT) and mesenchymal traits through ZEB1/2
Cell Death Discovery (2021)