Abstract
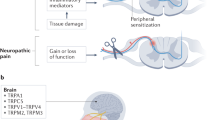
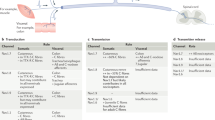
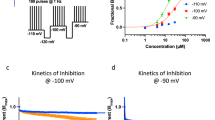
The transmission and processing of pain signals relies critically on the activities of ion channels that are expressed in afferent pain fibers. This includes voltage-gated channels, as well as background (or leak) channels that collectively regulate resting membrane potential and action potential firing properties. Dysregulated ion channel expression in response to nerve injury and inflammation results in enhanced neuronal excitability that underlies chronic neuropathic and inflammatory pain. Pharmacological modulators of ion channels, particularly those that target channels on peripheral neurons, are being pursued as possible analgesics. Over the past few years, a number of different types of ion channels have been implicated in afferent pain signaling. Here we give an overview of recent advances on sodium, calcium, potassium and chloride channels that are emerging as especially attractive targets for the treatment of pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bourinet, E. et al. Calcium permeable ion channels in pain signaling. Physiol. Rev. 94, 81–140 (2014).
Waxman, S.G. et al. Sodium channels and pain. Proc. Natl. Acad. Sci. USA 96, 7635–7639 (1999).
Dib-Hajj, S.D. et al. Gain-of-function mutation in NaV1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain 128, 1847–1854 (2005).
Faber, C.G. et al. Gain of function Nav1.7 mutations in idiopathic small fiber neuropathy. Ann. Neurol. 71, 26–39 (2012).
Faber, C.G. et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. USA 109, 19444–19449 (2012).
Okuse, K. et al. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 417, 653–656 (2002).
Toledo-Aral, J.J. et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc. Natl. Acad. Sci. USA 94, 1527–1532 (1997).
Rush, A.M. et al. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc. Natl. Acad. Sci. USA 103, 8245–8250 (2006).
Dib-Hajj, S.D. et al. The NaV1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 14, 49–62 (2013).
Nassar, M.A. et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 101, 12706–12711 (2004).
Shields, S.D. et al. Sodium channel Nav1.7 is essential for lowering heat pain threshold after burn injury. J. Neurosci. 32, 10819–10832 (2012).
Black, J.A. et al. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann. Neurol. 64, 644–653 (2008).
Yang, Y. et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 41, 171–174 (2004).
Cummins, T.R., Dib-Hajj, S.D. & Waxman, S.G. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J. Neurosci. 24, 8232–8236 (2004).
Fertleman, C.R. et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 52, 767–774 (2006).
Cox, J.J. et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature 444, 894–898 (2006).
Estacion, M. et al. A sodium channel gene SCN9A polymorphism that increases nociceptor excitability. Ann. Neurol. 66, 862–866 (2009).
Reimann, F. et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc. Natl. Acad. Sci. USA 107, 5148–5153 (2010).
Goldberg, Y.P. et al. Treatment of Nav1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain 153, 80–85 (2012).
Yang, Y. et al. Structural modelling and mutant cycle analysis predict pharmacoresponsiveness of a NaV1.7 mutant channel. Nat. Commun. 3, 1186 (2012).
Akopian, A.N., Sivilotti, L. & Wood, J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379, 257–262 (1996).
Renganathan, M., Cummins, T.R. & Waxman, S.G. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 86, 629–640 (2001).
Binshtok, A.M. et al. Nociceptors are interleukin-1β sensors. J. Neurosci. 28, 14062–14073 (2008).
Hudmon, A. et al. Phosphorylation of sodium channel Nav1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J. Neurosci. 28, 3190–3201 (2008).
Akopian, A.N. et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 2, 541–548 (1999).
Jarvis, M.F. et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA 104, 8520–8525 (2007).
Lai, J. et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain 95, 143–152 (2002).
Dib-Hajj, S. et al. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 25, 253–259 (2002).
Amaya, F. et al. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 26, 12852–12860 (2006).
Priest, B.T. et al. Contribution of the tetrodotoxin-resistant voltage-gated sodium channel NaV1.9 to sensory transmission and nociceptive behavior. Proc. Natl. Acad. Sci. USA 102, 9382–9387 (2005).
Craner, M.J. et al. Changes of sodium channel expression in experimental painful diabetic neuropathy. Ann. Neurol. 52, 786–792 (2002).
Cummins, T.R. et al. Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 21, 5952–5961 (2001).
Black, J.A. et al. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 82, 2776–2785 (1999).
Waxman, S.G., Kocsis, J.D. & Black, J.A. Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J. Neurophysiol. 72, 466–470 (1994).
Hains, B.C. et al. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J. Neurosci. 24, 4832–4839 (2004).
Lindia, J.A. et al. Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain 117, 145–153 (2005).
Samad, O.A. et al. Virus-mediated shRNA knockdown of Nav1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol. Ther. 21, 49–56 (2013).
Weiss, J. et al. Loss-of-function mutations in sodium channel Nav1.7 cause anosmia. Nature 472, 186–190 (2011).
Maljevic, S. & Lerche, H. Potassium channels: a review of broadening therapeutic possibilities for neurological diseases. J. Neurol. 260, 2201–2211 (2013).
Nockemann, D. et al. The K channel GIRK2 is both necessary and sufficient for peripheral opioid-mediated analgesia. EMBO Mol. Med. 5, 1263–1277 (2013).
Cao, X.H. et al. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J. Neurochem. 114, 1460–1475 (2010).
Rasband, M.N. et al. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. USA 98, 13373–13378 (2001).
Tsantoulas, C. et al. Sensory neuron downregulation of the Kv9.1 potassium channel subunit mediates neuropathic pain following nerve injury. J. Neurosci. 32, 17502–17513 (2012).
Zheng, Q. et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 154, 434–448 (2013).
Zhao, X. et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 16, 1024–1031 (2013).
Hao, J. et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 77, 899–914 (2013).
Xu, W. et al. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol. Pain 6, 49 (2010).
Klein, C.J. et al. Chronic pain as a manifestation of potassium channel-complex autoimmunity. Neurology 79, 1136–1144 (2012).
Honoré, E. The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261 (2007).
Marsh, B. et al. Leak K+ channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol. Cell. Neurosci. 49, 375–386 (2012).
Kang, D. & Kim, D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am. J. Physiol. Cell Physiol. 291, C138–C146 (2006).
Dobler, T. et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J. Physiol. (Lond.) 585, 867–879 (2007).
Tulleuda, A. et al. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol. Pain 7, 30 (2011).
Alloui, A. et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25, 2368–2376 (2006).
Noël, J. et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 28, 1308–1318 (2009).
Kang, D., Choe, C. & Kim, D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. (Lond.) 564, 103–116 (2005).
La, J.H. & Gebhart, G.F. Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G165–G174 (2011).
Cohen, A. et al. Pain-associated signals, acidosis and lysophosphatidic acid, modulate the neuronal K2P2.1 channel. Mol. Cell. Neurosci. 40, 382–389 (2009).
Zhou, J. et al. Intrathecal TRESK gene recombinant adenovirus attenuates spared nerve injury-induced neuropathic pain in rats. Neuroreport 24, 131–136 (2013).
Bagriantsev, S.N. et al. A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K channels. ACS Chem. Biol. 8, 1841–1851 (2013).
Berkefeld, H., Fakler, B. & Schulte, U. Ca2+-activated K+ channels: from protein complexes to function. Physiol. Rev. 90, 1437–1459 (2010).
Mongan, L.C. et al. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience 131, 161–175 (2005).
Li, W. et al. Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J. Cell. Physiol. 212, 348–357 (2007).
Bahia, P.K. et al. A functional role for small-conductance calcium-activated potassium channels in sensory pathways including nociceptive processes. J. Neurosci. 25, 3489–3498 (2005).
Zhang, X.L. et al. BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur. J. Neurosci. 31, 450–462 (2010).
Boettger, M.K. et al. Calcium-activated potassium channel SK1- and IK1-like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain 125, 252–263 (2002).
Chen, S.R., Cai, Y.Q. & Pan, H.L. Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J. Neurochem. 110, 352–362 (2009).
Cao, X.H. et al. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J. Neurochem. 121, 944–953 (2012).
Bhattacharjee, A. & Kaczmarek, L.K. For K+ channels, Na+ is the new Ca2+. Trends Neurosci. 28, 422–428 (2005).
Gao, S.B. et al. Slack and Slick KNa channels are required for the depolarizing afterpotential of acutely isolated, medium diameter rat dorsal root ganglion neurons. Acta Pharmacol. Sin. 29, 899–905 (2008).
Nuwer, M.O., Picchione, K.E. & Bhattacharjee, A. PKA-induced internalization of slack KNa channels produces dorsal root ganglion neuron hyperexcitability. J. Neurosci. 30, 14165–14172 (2010).
Huang, F. et al. TMEM16C facilitates Na-activated K currents in rat sensory neurons and regulates pain processing. Nat. Neurosci. 16, 1284–1290 (2013).
Bourinet, E. et al. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 24, 315–324 (2005).
Jagodic, M.M. et al. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J. Neurosci. 27, 3305–3316 (2007).
Francois, A. et al. State-dependent properties of a new T-type calcium channel blocker enhance CaV3.2 selectivity and support analgesic effects. Pain 154, 283–293 (2013).
Hildebrand, M.E. et al. A novel slow-inactivation-specific ion channel modulator attenuates neuropathic pain. Pain 152, 833–843 (2011).
Heppenstall, P.A. & Lewin, G.R. A role for T-type Ca2+ channels in mechanosensation. Cell Calcium 40, 165–174 (2006).
Rose, K.E. et al. Immunohistological demonstration of Ca3.2 T-type voltage-gated calcium channel expression in soma of dorsal root ganglion neurons and peripheral axons of rat and mouse. Neuroscience 250, 263–274 (2013).
Jacus, M.O. et al. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 32, 9374–9382 (2012).
Weiss, N. et al. A Cav3.2/syntaxin-1A signaling complex controls T-type channel activity and low-threshold exocytosis. J. Biol. Chem. 287, 2810–2818 (2012).
Tringham, E. et al. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci. Transl. Med. 4, 121ra19 (2012).
Hartzell, C., Putzier, I. & Arreola, J. Calcium-activated chloride channels. Annu. Rev. Physiol. 67, 719–758 (2005).
Kenyon, J.L. The reversal potential of Ca2+-activated Cl− currents indicates that chick sensory neurons accumulate intracellular Cl. Neurosci. Lett. 296, 9–12 (2000).
André, S. et al. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J. Neurophysiol. 90, 3764–3773 (2003).
Liu, B. et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl− channels. J. Clin. Invest. 120, 1240–1252 (2010).
Cho, H. et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 15, 1015–1021 (2012).
Benarroch, E.E. HCN channels: function and clinical implications. Neurology 80, 304–310 (2013).
Matsuyoshi, H. et al. Expression of hyperpolarization-activated cyclic nucleotide-gated cation channels in rat dorsal root ganglion neurons innervating urinary bladder. Brain Res. 1119, 115–123 (2006).
Wan, Y. Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain. Sheng Li Xue Bao 60, 579–580 (2008).
Papp, I., Hollo, K. & Antal, M. Plasticity of hyperpolarization-activated and cyclic nucleotid-gated cation channel subunit 2 expression in the spinal dorsal horn in inflammatory pain. Eur. J. Neurosci. 32, 1193–1201 (2010).
Weng, X. et al. Chronic inflammatory pain is associated with increased excitability and hyperpolarization-activated current (Ih) in C- but not Aδ-nociceptors. Pain 153, 900–914 (2012).
Acosta, C. et al. HCN1 and HCN2 in rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression. PLoS ONE 7, e50442 (2012).
Emery, E.C. et al. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science 333, 1462–1466 (2011).
Descoeur, J. et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 3, 266–278 (2011).
Wickenden, A.D., Maher, M.P. & Chaplan, S.R. HCN pacemaker channels and pain: a drug discovery perspective. Curr. Pharm. Des. 15, 2149–2168 (2009).
Lafrenière, R.G. et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 16, 1157–1160 (2010).
Rehak, R. et al. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS ONE 8, e61844 (2013).
Kuisle, M. et al. Functional stabilization of weakened thalamic pacemaker channel regulation in rat absence epilepsy. J. Physiol. (Lond.) 575, 83–100 (2006).
Liu, X.J. et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat. Med. 14, 1325–1332 (2008).
Hoeijmakers, J.G. et al. Small nerve fibres, small hands and small feet: a new syndrome of pain, dysautonomia and acromesomelia in a kindred with a novel NaV1.7 mutation. Brain 135, 345–358 (2012).
Acknowledgements
We thank C. Midgley for editorial assistance. G.W.Z. is supported by a Canada Research Chair award, an Alberta Innovates – Health Solutions Scientist award and grants from the Canadian Institutes of Health Research. S.G.W. is supported by the Bridget M. Flaherty Professorship in Neurology, Neurobiology and Pharmacology at Yale and is supported by grants from the Rehabilitation Research Service and Biomedical Laboratory Research Service, US Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Waxman, S., Zamponi, G. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 17, 153–163 (2014). https://doi.org/10.1038/nn.3602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3602
This article is cited by
-
Trace amine-associated receptor 1 regulation of Kv1.4 channels in trigeminal ganglion neurons contributes to nociceptive behaviors
The Journal of Headache and Pain (2023)
-
Gene therapy for chronic pain: emerging opportunities in target-rich peripheral nociceptors
Nature Reviews Neuroscience (2023)
-
Central and peripheral contributions of T-type calcium channels in pain
Molecular Brain (2022)
-
Transcriptomic analysis of human sensory neurons in painful diabetic neuropathy reveals inflammation and neuronal loss
Scientific Reports (2022)
-
Regulation of CaV3.2 channels by the receptor for activated C kinase 1 (Rack-1)
Pflügers Archiv - European Journal of Physiology (2022)