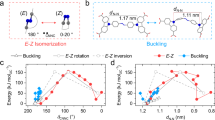
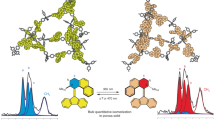
Abstract
Porous compounds are ubiquitous and indispensable in daily life as adsorbents and catalysts. The discovery of a new porous compound with unique properties based on intrinsic nanosized space and surface functionalities is scientifically and technologically important. However, the functional species used in this context are limited to those that are sufficiently inert to not spoil the porous structures. Here, we show a new strategy to achieve a crystalline porous material with the pore surface regularly decorated with highly reactive ‘bare’ nitrenes that are photonically generated from stable ‘dormant’ precursors at will. The bare triplet nitrenes were accessible to and reacted with adsorbed oxygen or carbon monoxide molecules, which showed not only activation of the pore surface, but also a high probability of chemical trapping and conversion of guest molecules by light stimulation on demand.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schüth, F., Sing, K. S. W. & Weitkamp, J. Handbook of Porous Solids (Wiley-VCH, 2002).
Rowsell, J. L. C., Spencer, E. C., Eckert, J., Howard, J. A. K. & Yaghi, O. M. Gas adsorption sites in a large-pore metal–organic framework. Science 309, 1350–1354 (2005).
Serre, C. et al. Role of solvent–host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007).
Yang, Q., Kapoor, M. P. & Inagaki, S. Sulfonic acid-functionalized mesoporous benzene–silica with a molecular-scale periodicity in the walls. J. Am. Chem. Soc. 124, 9694–9695 (2002).
Alauzun, J., Mehdi, A., Reyé, C. & Corriu, R. J. P. Mesoporous materials with an acidic framework and basic pores. A successful cohabitation. J. Am. Chem. Soc. 128, 8718–8719 (2006).
Georgiev, P. A., Albinati, A., Mojet, B. L., Ollivier, J. & Eckert, J. Observation of exceptionally strong binding of molecular hydrogen in a porous material: Formation of an η2-H2 complex in a Cu-exchanged ZSM-5 zeolite. J. Am. Chem. Soc. 129, 8086–8087 (2007).
Maji, T. K., Matsuda, R. & Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nature Mater. 6, 142–148 (2007).
Asefa, T., MacLachlan, M. J., Coombs, N. & Ozin, G. A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 402, 867–871 (1999).
Feng, X. et al. Functionalized monolayers on ordered mesoporous supports. Science 276, 923–926 (1997).
Li, Q. et al. Docking in metal–organic frameworks. Science 325, 855–859 (2009).
Matsuda, R. et al. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature 436, 238–241 (2005).
Xiang, S., Zhang, Y., Xin, Q. & Li, C. Asymmetric epoxidation of allyl alcohol on organic–inorganic hybrid chiral catalysts grafted onto the surface of silica and in the mesopores of MCM-41. Angew. Chem. Int. Ed. 41, 821–824 (2002).
Che, S. et al. A novel anionic surfactant templating route for synthesizing mesoporous silica with unique structure. Nature Mater. 2, 801–805 (2003).
Bradshaw, D., Claridge, J. B., Cussen, E. J., Prior, T. J. & Rosseinsky, M. J. Design, chirality, and flexibility in nanoporous molecule-based materials. Acc. Chem. Res. 38, 273–282 (2005).
Kuschel, A., Sievers, H. & Polarz, S. Amino acid silica hybrid materials with mesoporous structure and enantiopure surfaces. Angew. Chem. Int. Ed. 47, 9513–9517 (2008).
Moss, R. A., Platz, M. S. & Jones, M. Jr Reactive Intermediate Chemistry (Wiley-Interscience, 2004).
Yaghi, O. M. et al. Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003).
Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 37, 191–214 (2008).
Dalgarno, S. J., Power, N. P. & Atwood, J. L. Metallo-supramolecular capsules. Coord. Chem. Rev. 252, 825–841 (2008).
Morris, R. E. & Wheatley, P. S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 47, 4966–4981 (2008).
Nouar, F. et al. Supermolecular building blocks (SBBs) for the design and synthesis of highly porous metal–organic frameworks. J. Am. Chem. Soc. 130, 1833–1835 (2008).
Kitagawa, S., Kitaura, R. & Noro, S-i. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Yang, S. et al. Cation-induced kinetic trapping and enhanced hydrogen adsorption in a modulated anionic metal–organic framework. Nature Chem. 1, 487–493 (2009).
Mulfort, K. L. & Hupp, J. T. Chemical reduction of metal–organic framework materials as a method to enhance gas uptake and binding. J. Am. Chem. Soc. 129, 9604–9605 (2007).
Halder, G. J., Kepert, C. J., Moubaraki, B., Murray, K. S. & Cashion, J. D. Guest-dependent spin crossover in a nanoporous molecular framework material. Science 298, 1762–1765 (2002).
Dincaˇ, M. & Long, J. R. High-enthalpy hydrogen adsorption in cation-exchanged variants of the microporous metal–organic framework Mn3[(Mn4Cl)3(BTT)8(CH3OH)10]2 . J. Am. Chem. Soc. 129, 11172–11176 (2007).
Lee, Y-G., Moon, H. R., Cheon, Y. E. & Suh, M. P. A comparison of the H2 sorption capacities of isostructural metal–organic frameworks with and without accessible metal sites: [{Zn2(abtc)(dmf)2}3] and [{Cu2(abtc)(dmf)2}3] versus [{Cu2(abtc)}3]. Angew. Chem. Int. Ed. 47, 7741–7745 (2008).
Bradshaw, D., Warren, J. E. & Rosseinsky, M. J. Reversible concerted ligand substitution at alternating metal sites in an extended solid. Science 315, 977–980 (2007).
Seo, J. S. et al. A homochiral metal–organic porous material for enantioselective separation and catalysis. Nature 404, 982–986 (2000).
Hasegawa, S. et al. Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: Selective sorption and catalysis. J. Am. Chem. Soc. 129, 2607–2614 (2007).
Ingleson, M. J. et al. Generation of a solid Brønsted acid site in a chiral framework. Chem. Commun. 1287–1289 (2008).
Kawamichi, T., Haneda, T., Kawano, M. & Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 461, 633–635 (2009).
Wang, Z. & Cohen, S. M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 38, 1315–1329 (2009).
Seo, J., Matsuda, R., Sakamoto, H., Bonneau, C. & Kitagawa, S. A pillared-layer coordination polymer with a rotatable pillar acting as a molecular gate for guest molecules. J. Am. Chem. Soc. 131, 12792–12800 (2009).
Gritsan, N. P. & Platz, M. S. Kinetics, spectroscopy, and computational chemistry of arylnitrenes. Chem. Rev. 106, 3844–3867 (2006).
Sasaki, A., Mahé, L., Izuoka, A. & Sugawara, T. Chemical consequences of arylnitrenes in the crystalline environment. Bull. Chem. Soc. Jpn 71, 1259–1275 (1998).
Harder, T., Wessig, P., Bendig, J. & Stösser, R. Photochemical reactions of nitroso oxides at low temperatures: The first experimental evidence for dioxaziridines. J. Am. Chem. Soc. 121, 6580–6588 (1999).
Inui, H., Irisawa, M. & Oishi, S. Reaction of (4-nitrophenyl)nitrene with molecular oxygen in low-temperature matrices: First IR detection and photochemistry of aryl nitroso oxide. Chem. Lett. 34, 478–479 (2005).
Dunkin, I. R. & Thomson, P. C. P. Pentafluorophenyl nitrene: A matrix isolated aryl nitrene that does not undergo ring expansion. J. Chem. Soc. Chem. Commun. 1192–1193 (1982).
Lamara, K. & Smalley, R. K. 3H-Azepines and related systems. Part 4. Preparation of 3H-azepin-2-ones and 6H-azepino[2,1-b]quinazolin-12-ones by photo-induced ring expansions of aryl azides. Tetrahedron 47, 2277–2290 (1991).
Priewisch, B. & Rueck-Braun, K. Efficient preparation of nitrosoarenes for the synthesis of azobenzenes. J. Org. Chem. 70, 2350–2352 (2005).
Horike, S., Tanaka, D., Nakagawa, K. & Kitagawa, S. Selective guest sorption in an interdigitated porous framework with hydrophobic pore surfaces. Chem. Commun. 3395–3397 (2007).
Acknowledgements
The synchrotron radiation experiments were carried out at BL02B1 in SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal no. 2009A1569).
Author information
Authors and Affiliations
Contributions
H.S., R.M. and S.K. conceived the project. H.S. and R.M. prepared and analysed all compounds described and carried out the sorption, spectroscopic measurements and photochemical experiments. K.S. and M.T. assisted the crystallographic study using synchrotron X-rays. H.S., R.M. and S.K. designed the study, analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2102 kb)
Rights and permissions
About this article
Cite this article
Sato, H., Matsuda, R., Sugimoto, K. et al. Photoactivation of a nanoporous crystal for on-demand guest trapping and conversion. Nature Mater 9, 661–666 (2010). https://doi.org/10.1038/nmat2808
Issue Date:
DOI: https://doi.org/10.1038/nmat2808
This article is cited by
-
Self-catalysed aerobic oxidization of organic linker in porous crystal for on-demand regulation of sorption behaviours
Nature Communications (2015)
-
Ab initio design of nanostructures for solar energy conversion: a case study on silicon nitride nanowire
Nanoscale Research Letters (2014)
-
Microimaging of transient guest profiles to monitor mass transfer in nanoporous materials
Nature Materials (2014)
-
Confinement of pyridinium hemicyanine dye within an anionic metal-organic framework for two-photon-pumped lasing
Nature Communications (2013)
-
Localized cell stimulation by nitric oxide using a photoactive porous coordination polymer platform
Nature Communications (2013)