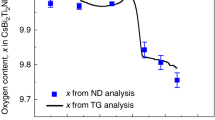
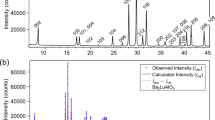
Abstract
Some oxides contain sufficient equilibrium concentrations of protons in wet atmospheres to show useful proton conduction at elevated temperatures1. As an example, Y-doped BaCeO3 has shown promising performance as a thin-film electrolyte in fuel cells at intermediate temperatures (400–600 ∘C)2. In contrast to proton-conducting polymers (for example, Nafion(R)) and acid salts (for example, CsHSO4), such oxidic ceramics are stable at sufficiently elevated temperatures that electrode kinetics are fast and insensitive to poisoning, but they tend to be basic (Ba-based or Sr-based) compounds with poor chemical and mechanical stability3. In search of more stable proton-conducting materials, we have investigated several acceptor-doped rare-earth ortho-niobates and ortho-tantalates, RE1−xAxMO4 (M=Nb,Ta). We show that this class of materials shows mixed protonic, native ionic and electronic conduction depending on conditions. Both the low-temperature monoclinic and high-temperature tetragonal polymorphs show proton conduction. The proton conductivity is dominant in wet atmospheres below roughly 800∘C and the highest proton conductivity of approximately 10−3Scm−1 was found for Ca-doped LaNbO4. These transport characteristics can be used in sensors and fuel cells provided that the electrolyte film thickness is in the micrometre range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Iwahara, H., Esaka, T., Uchida, H. & Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 3–4, 359–364 (1981).
Ito, N., Iijima, M., Kimura, K. & Iguchi, S. New intermediate temperature fuel cell with ultra-thin proton conductor electrolyte. J. Power Sources 152, 200–203 (2005).
Kreuer, K.-D. On the development of proton conducting materials for technological applications. Solid State Ion. 97, 1–15 (1997).
Shimura, T., Tokiwa, Y. & Iwahara, H. Protonic conduction in lanthanum strontium aluminate and lanthanum niobate-based oxides at elevated temperatures. Solid State Ion. 154–155, 653–658 (2002).
Gingerich, K. A. & Bair, H. E. Relation between ionic radii and transformation temperature in rare earth niobates. Adv. X-Ray Anal. 7, 22–30 (1964).
Bohn, H. G. & Schober, T. Electrical conductivity of the high-temperature proton conductor BaZr0.9Y0.1O2.95 . J. Am. Ceram. Soc. 83, 768–772 (2000).
Larring, Y. & Norby, T. The equilibrium between oxygen vacancies, water vapour and protons in rare earth oxides. Solid State Ion. 97, 523–528 (1997).
Kitamura, N., Amezawa, K., Tomii, Y., Yamamoto, N. & Hanada, T. Protonic conduction in Sr-doped (La1−xSmx)PO4 . Solid State Ion. 175, 563–567 (2004).
Kreuer, K.-D. On the complexity of proton conduction phenomena. Solid State Ion. 136–137, 149–160 (2000).
Prytz, O. & Tafto, J. Accurate determination of domain boundary orientation in LaNbO4 . Acta Mater. 53, 297–302 (2005).
Norby, T. EMF method determination of conductivity contributions from protons and other foreign ions in oxides. Solid State Ion. 28–30, 1586–1591 (1988).
Sutija, D., Norby, T. & Björnbom, P. Transport number determinations by the concentration cell/open-circuit voltage method for oxides with mixed electronic, ionic, and protonic conductivity. Solid State Ion. 77, 167–174 (1995).
Acknowledgements
This work has been supported by the EU GROWTH ‘CERHYSEP’ project (G1RD-CT-2001-00651) and the Nanomat project of the Research Council of Norway (RCN, Grant No. 15851/431; Functional Oxides for Energy Technology).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Haugsrud, R., Norby, T. Proton conduction in rare-earth ortho-niobates and ortho-tantalates. Nature Mater 5, 193–196 (2006). https://doi.org/10.1038/nmat1591
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1591
This article is cited by
-
Sol–gel derived amorphous LaNbOx films for forming-free RRAM applications
Applied Physics A (2024)
-
High proton conduction in Ba2LuAlO5 with highly oxygen-deficient layers
Communications Materials (2023)
-
High proton conductivity within the ‘Norby gap’ by stabilizing a perovskite with disordered intrinsic oxygen vacancies
Nature Communications (2023)
-
Semiconductor Electrochemistry for Clean Energy Conversion and Storage
Electrochemical Energy Reviews (2021)
-
High oxide ion and proton conductivity in a disordered hexagonal perovskite
Nature Materials (2020)