Abstract
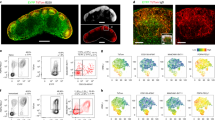
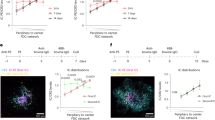
The splenic marginal zone is a site of blood flow, and the specialized B cell population that inhabits this compartment has been linked to the capture and follicular delivery of blood-borne antigens. However, the mechanism of this antigen transport has remained unknown. Here we show that marginal zone B cells were not confined to the marginal zone but continuously shuttled between the marginal zone and follicular areas, such that many of the cells visited a follicle every few hours. Migration to the follicle required the chemokine receptor CXCR5, whereas return to the marginal zone was promoted by the sphingosine 1-phosphate receptors S1P1 and S1P3. Treatment with an S1P1 antagonist caused displacement of marginal zone B cells from the marginal zone. Marginal zone–follicle shuttling of marginal zone B cells provides an efficient mechanism for systemic antigen capture and delivery to follicular dendritic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cyster, J.G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005).
MacLennan, I.C.M., Gray, D., Kumararatne, D.S. & Bazin, H. The lymphocytes of splenic marginal zones: a distinct B-cell lineage. Immunol. Today 3, 305–307 (1982).
Martin, F. & Kearney, J.F. Marginal-zone B cells. Nat. Rev. Immunol. 2, 323–335 (2002).
Kraal, G. Cells in the marginal zone of the spleen. Int. Rev. Cytol. 132, 31–73 (1992).
Nossal, G.J., Austin, C.M., Pye, J. & Mitchell, J. Antigens in immunity. XII. Antigen trapping in the spleen. Int. Arch. Allergy Appl. Immunol. 29, 368–383 (1966).
Mitchell, J. & Abbot, A. Antigens in immunity. XVI. A light and electron microscope study of antigen localization in the rat spleen. Immunology 21, 207–224 (1971).
Brown, J.C., Harris, G., Papamichail, M., Sljivic, V.S. & Holborow, E.J. The localization of aggregated human γ-globulin in the spleens of normal mice. Immunology 24, 955–968 (1973).
Gray, D., Kumararatne, D.S., Lortan, J., Khan, M. & MacLennan, I.C. Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology 52, 659–669 (1984).
Groeneveld, P.H., Erich, T. & Kraal, G. The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology 58, 285–290 (1986).
Ferguson, A.R., Youd, M.E. & Corley, R.B. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int. Immunol. 16, 1411–1422 (2004).
Cinamon, G. et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5, 713–720 (2004).
Vora, K.A. et al. Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J. Leukoc. Biol. 78, 471–480 (2005).
Schwab, S.R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Rosen, H. & Goetzl, E.J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5, 560–570 (2005).
Rosen, H., Sanna, M.G., Cahalan, S.M. & Gonzalez-Cabrera, P.J. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 28, 102–107 (2007).
Lo, C.G., Xu, Y., Proia, R.L. & Cyster, J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201, 291–301 (2005).
Foss, F.W., Jr. et al. Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists. Bioorg. Med. Chem. 15, 663–667 (2007).
Lo, C.G., Lu, T.T. & Cyster, J.G. Integrin-dependence of lymphocyte entry into the splenic white pulp. J. Exp. Med. 197, 353–361 (2003).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J.V. Absence of marginal zone B cells in Pyk-2 deficient mice define their role in the humoral response. Nat. Immunol. 1, 31–36 (2000).
Sanchez, T. et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278, 47281–47290 (2003).
Allende, M.L., Dreier, J.L., Mandala, S. & Proia, R.L. Expression of the sphingosine-1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004).
Rickert, R.C., Rajewsky, K. & Roes, J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376, 352–355 (1995).
Martin, F. & Kearney, J.F. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity 12, 39–49 (2000).
Forster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Ansel, K.M. et al. A chemokine driven positive feedback loop organizes lymphoid follicles. Nature 406, 309–314 (2000).
Gretz, J.E., Norbury, C.C., Anderson, A.O., Proudfoot, A.E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Nolte, M.A. et al. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 198, 505–512 (2003).
Whipple, E.C., Shanahan, R.S., Ditto, A.H., Taylor, R.P. & Lindorfer, M.A. Analyses of the in vivo trafficking of stoichiometric doses of an anti-complement receptor 1/2 monoclonal antibody infused intravenously in mice. J. Immunol. 173, 2297–2306 (2004).
Nolte, M.A., Hoen, E.N., van Stijn, A., Kraal, G. & Mebius, R.E. Isolation of the intact white pulp. Quantitative and qualitative analysis of the cellular composition of the splenic compartments. Eur. J. Immunol. 30, 626–634 (2000).
Taylor, P.R. et al. The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur. J. Immunol. 32, 1888–1896 (2002).
Pasparakis, M., Kousteni, S., Peschon, J. & Kollias, G. Tumor necrosis factor and the p55TNF receptor are required for optimal development of the marginal sinus and for migration of follicular dendritic cell precursors into splenic follicles. Cell. Immunol. 201, 33–41 (2000).
Voigt, I. et al. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur. J. Immunol. 30, 560–567 (2000).
Girkontaite, I. et al. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J. Exp. Med. 200, 1491–1501 (2004).
Gunn, M.D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature 391, 799–803 (1998).
Ngo, V.N. et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189, 403–412 (1999).
Lu, T.T. & Cyster, J.G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297, 409–412 (2002).
Woolf, E. et al. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol. 8, 1076–1085 (2007).
Liu, C.H. et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 (1999).
Oo, M.L. et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists Induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282, 9082–9089 (2007).
Gonzalez-Cabrera, P.J., Hla, T. & Rosen, H. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J. Biol. Chem. 282, 7254–7264 (2007).
Veerman, A.J. & van Rooijen, N. Lymphocyte capping and lymphocyte migration as associated events in the in vivo antigen trapping process. An electron-microscopic autoradiographic study in the spleen of mice. Cell Tissue Res. 161, 211–217 (1975).
Phan, T.G., Grigorova, I., Okada, T. & Cyster, J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 8, 992–1000 (2007).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000).
Molina, H. et al. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc. Natl. Acad. Sci. USA 93, 3357–3361 (1996).
Ansel, K.M., Harris, R.B. & Cyster, J.G. CXCL13 Is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 16, 67–76 (2002).
Förster, R. et al. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87, 1037–1047 (1996).
Kono, M. et al. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279, 29367–29373 (2004).
Allen, C.D., Okada, T., Tang, H.L. & Cyster, J.G. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531 (2007).
Acknowledgements
We thank J. Pereira for sharing in vivo labeling techniques; J. An and Y. Xu for technical help; R. Proia (National Institute of Diabetes and Digestive and Kidney Diseases) for S1P1- and S1P3-deficient mice; M. Lipp (Max Delbrück Center of Molecular Medicine;) and R. Forster (Hannover Medical School) for CXCR5-deficient mice; X. Wu and J. Atkinson (Washington University School of Medicine) for Cr2−/− mice; K. Rajewsky (The CBR Institute for Biomedical Research, Harvard Medical School) for CD19-Cre mice; K. Lynch and T. MacDonald (University of Virginia Medical Center) for VPC44116; C. Allen for help with the LSRII; L. Shiow for discussions; and T. Phan for comments on the manuscript. Supported by the Howard Hughes Medical Institute (G.C. and J.G.C.) and the National Institutes of Health (AI40098).
Author information
Authors and Affiliations
Contributions
G.C. and J.G.C. designed and conceptualized the research; G.C. did the experiments; M.A.Z. was involved in several in vivo labeling experiments; O.M.L. helped with animal management and genotyping; F.W.F. generated VPC44116; and G.C. and J.G.C. analyzed the data and prepared the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Cinamon, G., Zachariah, M., Lam, O. et al. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol 9, 54–62 (2008). https://doi.org/10.1038/ni1542
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1542
This article is cited by
-
Dynamic encounters with red blood cells trigger splenic marginal zone B cell retention and function
Nature Immunology (2024)
-
CXCL13 plasma levels function as a biomarker for disease activity in patients with chronic lymphocytic leukemia
Leukemia (2021)
-
Notch2-mediated plasticity between marginal zone and follicular B cells
Nature Communications (2021)
-
The human spleen as the center of the blood defense system
International Journal of Hematology (2020)
-
Innate B Cells: the Archetype of Protective Immune Cells
Clinical Reviews in Allergy & Immunology (2020)