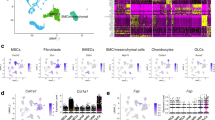
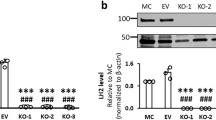
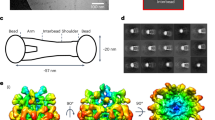
Abstract
The tissue–specific organization of collagen molecules into tridimensional macroaggregates determines the physiomechanical properties of most connective tissues, but the factors and mechanisms controlling this process are unknown. It has been postulated that quantitatively minor types V and XI collagen regulate the growth of type I and II collagen fibrils, respectively. To test this hypothesis, we created mice that produce a structurally abnormal α2(V) collagen chain. Homozygous mutant mice survive poorly, possibly because of complications from spinal deformities, and exhibit skin and eye abnormalities caused by disorganized type I collagen fibrils. Our results demonstrate that type V collagen is a key determinant in the assembly of tissue–specific matrices, and provide an animal model for human connective tissue disorders
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Prockop, D.J. Mutations that alter the primary structure of type I collagen. J. biol. Chem. 265, 15349–15352 (1990).
Birk, D.E., Silver, F.H. & Trelstad, R.L., Martrix Assembly. in Cell Biology of Extracellular Matrix (ed. Hay, E.D.) 221–254 (Plenum Press, New York, 1991).
Birk, D.E., Fitch, J.M., Babiarz, J.P., Doane, K.J. & Linsenmayer, T.F. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J. cell. Sci. 95, 649–657 (1990).
Fäsaler, R. et al. Mice lacking α1(IX)collagen develop noninflamatory degenerative joint disease. Proc. natn. Acad. Sci. U.S.A 91, 5070–6074 (1994).
Rosati, R. et al. Normal long bone growth and development in type X collagen-null mice. Nature Genet. 8, 129–135 (1994).
Vuorio, E. & de Crombrugghe, B. The family of collagen genes. A. Rev. Biochem. 59, 837–872 (1990).
Rook, A. & Dawber, R., Diseases of the Hair and Scalp. (Blackwell Scientific. Oxford, 1982).
Hay, E.D. Development of the vertebrate cornea. Int. Rev. Cytol. 63, 263–322 (1979).
Birk, D.E. & Trelstad, R.L. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J. Cell Biol. 99, 2024–2033 (1984).
Tseng, S.C.G., Smuckler, D. & Stern, R. Comparison of collagen types in adult and fetal bovine corneas. J. biol. Chem. 257, 2627–2633 (1982).
Birk, D.E., Fitch, J.M., Babiarz, J.P. & Linsenmayer, T.F. Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol. 106, 999–1008 (1988).
Linsenmayer, T.F. et al. Type V collagen: molecular structure and fibrillar organization of the chicken α1(V) NH2-terminal domain, a putative regulator of corneal fibrillogenesis. J. Cell Biol. 121, 1181–1189 (1993).
Moradi-Améli, M. et al. Diversity in the processing events at the N-terminus of collagen V. Eur. J. Biochem. 221, 987–995 (1994).
Bonadio, J. et al. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc. natn. Acad. Sci. U.S.A. 87, 7145–7149 (1990).
Andrikopoulos, K., Suzuki, H.R., Solursh, M. & Ramirez, F. Localization of pro-α2(V) collagen transcripts in the tissues of the developing mouse embryo. Dev. Dyn. 195, 113–120 (1992).
Saga, Y., Yagi, T., Ikawa, Y., Sakakura, T. & Aizawa, S. Mice develop normally without tenascin. Genes Dev. 6, 1821–1831 (1992).
Kratochwil, K., Dziadek, M., Lohler, J., Harbers, K. & Jaenisch, R. Normal epithelial branching morphogenesis in the absence of collagen I. Dev. Biol. 117, 596–606 (1986).
Byers, P.H. & Holbrook, K.A. Ehlers-Danlos Syndrome. in Principles and practice of medical genetics (eds Emery. A.E.H. & Rimoin, D.L.) 1065–1081 (Churchill Livingstone, Edinburgh, 1990).
Li, Y. et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell (In the press).
Tybulewicz, V.L.J., Crawford, C.E., Jackson, P.K., Bronson, R.T. & Mulligan, R.C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl protooncogene. Cell 65, 1153–1163 (1991).
Li, E., Bestor, T.H. & Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 69, 915–926 (1992).
Bradley, A. Production and analysis of chimearic mice. In Teratocarcinomas and embryonic stem cells: a practical approach (ed. Robertson, E.J.) 113–151 (IRL Press, Oxford, 1987).
Sambrook, E., Fritsch, E.F. & Maniatis, T. Molecular cloning: a laboratory manual 1–545 (Cold Spring Harbor, New York, 1989).
Broek, D.L., Madri, J., Elkenberry, E.F. & Brodsky, B. Characterization of the tissue form of type V collagen from chick bone. J. biol. Chem. 260, 555–562 (1985).
Woodbury, D., Benson, C.V. & Ramirez, F. Amino-terminal propeptide of human pro-α2(V) collagen conforms to the structural criteria of a fibrillar procoliagen molecule. J. biol. Chem. 284, 2735–2738 (1989).
Glorieux, F.H., Salle, B.L., Travers, R. & Audra, P.H. Dynamic histomorphometric evaluation of human fetal bone formation. Bone 12, 377–381 (1991).
Lufkin, T., Mark, M., Hart, C.P., LeMeur, M. & Chambon, P. Disruption of the Hox-1.6 homeobox gene results in defects in a region corresponding to its postral domain of expression. Cell 66, 1105–1119 (1992).
Keene, D.R., Engvall, E. & Glanville, R.W. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J. Cell Biol. 107, 1995–2006 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andrikopoulos, K., Liu, X., Keene, D. et al. Targeted mutation in the col5a2 gene reveals a regulatory role for type V collagen during matrix assembly. Nat Genet 9, 31–36 (1995). https://doi.org/10.1038/ng0195-31
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0195-31