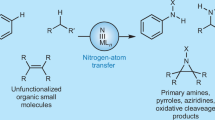
Abstract
C–H bond oxidation reactions underscore the existing paradigm wherein high reactivity and high selectivity are inversely correlated. The development of catalysts capable of oxidizing strong aliphatic C(sp3)–H bonds while displaying chemoselectivity (that is, tolerance of more oxidizable functionality) remains an unsolved problem. Here, we describe a catalyst, manganese tert-butylphthalocyanine [Mn(tBuPc)], that is an outlier to the reactivity–selectivity paradigm. It is unique in its capacity to functionalize all types of C(sp3)–H bond intramolecularly, while displaying excellent chemoselectivity in the presence of π functionality. Mechanistic studies indicate that [Mn(tBuPc)] transfers bound nitrenes to C(sp3)–H bonds via a pathway that lies between concerted C–H insertion, observed with reactive noble metals such as rhodium, and stepwise radical C–H abstraction/rebound, as observed with chemoselective base metals such as iron. Rather than achieving a blending of effects, [Mn(tBuPc)] aminates even 1° aliphatic and propargylic C–H bonds, demonstrating reactivity and selectivity unusual for previously known catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roizen, J. L., Harvey, M. E. & Du Bois, J. Metal-catalyzed nitrogen-atom transfer methods for the oxidation of aliphatic C–H bonds. Acc. Chem. Res. 45, 911–922 (2012).
White, M. C. Adding aliphatic C–H bond oxidations to synthesis. Science 335, 807–809 (2012).
Que, L. Jr. The road to non-heme oxoferryls and beyond. Acc. Chem. Res. 40, 493–500 (2007).
Nam, W., Lee, Y. & Fukuzumi, S. Tuning reactivity and mechanism in oxidation reactions by mononuclear nonheme iron(IV)–oxo complexes. Acc. Chem. Res. 47, 1146–1154 (2014).
Chen, M. S. & White, M. C. A predictable selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Gormisky, P. E. & White, M. C. Catalyst-controlled aliphatic C–H oxidations with a predictive model for site-selectivity. J. Am. Chem. Soc. 135, 14052–14055 (2013).
Jeffrey, J. L. & Sarpong, R. Intramolecular C(sp3)–H amination. Chem. Sci. 4, 4092–4106 (2013).
Lebel, H. in Catalyzed Carbon–Heteroatom Bond Formation (ed. Yudin, A. K.) 137–155 (Wiley-VCH, 2011).
Collet, F., Dodd, R. H. & Dauban, P. Catalytic C–H amination: recent progress and future directions. Chem. Commun. 5061–5074 (2009).
Che, C. M., Lo, V. K. Y., Zhou, C. Y. & Huang, J. S. Selective functionalization of saturated C–H bonds with metalloporphyrin catalysts. Chem. Soc. Rev. 40, 1950–1975 (2011).
Paradine, S. M. & White, M. C. Iron-catalyzed intramolecular allylic C–H amination. J. Am. Chem. Soc. 134, 2036–2039 (2012).
Hennessy, E. T. & Betley, T. A. Complex N-heterocycle synthesis via iron-catalyzed, direct C–H bond amination. Science 340, 591–595 (2013).
Hennessy, E. T., Liu, R. Y., Iovan, D. A., Duncan, R. A. & Betley, T. A. Iron-mediated intermolecular N-group transfer chemistry with olefinic substrates. Chem. Sci. 5, 1526–1532 (2014).
Liu, Y. et al. Nonheme iron-mediated amination of C(sp3)–H bonds. Quinquepyridine-supported iron-imide/nitrene intermediates by experimental studies and DFT calculations. J. Am. Chem. Soc. 135, 7194–7204 (2013).
Harvey, M. E., Musaev, D. G. & Du Bois, J. A diruthenium catalyst for selective, intramolecular allylic C–H amination: reaction development and mechanistic insight gained through experiment and theory. J. Am. Chem. Soc. 133, 17207–17216 (2011).
Suzuki, K., Oldenburg, P. D. & Que, L. Jr. Iron-catalyzed asymmetric olefin cis-dihydroxylation with 97% enantiomeric excess. Angew. Chem. Int. Ed. 47, 1887–1889 (2008).
Friedfel, M. R. et al. Cobalt precursors for high-throughput discovery of base metal asymmetric alkene hydrogenation catalysts. Science 342, 1076–1080 (2013).
Jagadeesh, R. V. et al. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 342, 1073–1076 (2013).
Zuo, W., Lough, A. J., Young, F. L. & Morris, R. H. Amine(imine)diphosphine iron catalysts for asymmetric transfer hydrogenation of ketones and imines. Science 342, 1080–1083 (2013).
Nappa, M. J. & McKinney, R. J. Selectivity control by axial ligand modification in manganese porphyrin catalyzed oxidations. Inorg. Chem. 27, 3740–3745 (1988).
Zalatan, D. N. & Du Bois, J. Oxidative cyclization of sulfamate esters using NaOCl—a metal-mediated Hoffman–Löffler–Freytag reaction. Synlett. 143–146 (2009).
Groves, J. T., Kruper, W. J. & Haushalter, R. C. Hydrocarbon oxidations with oxometalloporphinates. Isolation and reactions of a (porphinato)manganese(V) complex. J. Am. Chem. Soc. 102, 6375–6377 (1980).
Cook, B. R., Reinert, T. J. & Suslick, K. S. Shape-selective alkane hydroxylation by metalloporphyrin catalysts. J. Am. Chem. Soc. 108, 7281–7286 (1986).
Hill, C. J. & Hollander, F. J. Structural characterization of a complex of manganese(V) nitrido[tetrakis(p-methoxyphenyl)porphinato]manganese(V). J. Am. Chem. Soc. 104, 7318–7319 (1982).
Mehn, M. P. & Peters, J. C. Mid- to high-valent imido and nitrido complexes of iron. J. Inorg. Biochem. 100, 634–643 (2006).
Jensen, M. P., Mehn, M. P. & Que, L. Jr. Intramolecular aromatic amination through iron-mediated nitrene transfer. Angew. Chem. Int. Ed. 42, 4357–4360 (2003).
Luo, Y.-R. & Cheng, J.-P. in CRC Handbook of Chemistry & Physics 94th edn (ed. Haynes, W. M.) 9–65 (CRC, 2013).
Breslow, R. & Gellman, S. H. Intramolecular nitrene C–H insertions mediated by transition-metal complexes as nitrogen analogues of cytochrome P-450 reactions. J. Am. Chem. Soc. 105, 6728–6729 (1983).
Lever, A. B. P. & Wilshire, J. P. Redox potentials of metal phthalocyanines in non-aqueous media. Can. J. Chem. 54, 2514–2516 (1976).
Löbbert, G. in Ullmann’s Encyclopedia of Industrial Chemistry Vol. 27 (ed. Löbbert, G.) 181–213 (Wiley-VCH, 2012).
Fiori, K. W., Espino, C. G., Brodsky, B. H. & Du Bois, J. A mechanistic analysis of the Rh-catalyzed intramolecular C–H amination reaction. Tetrahedron. 65, 3042–3051 (2009).
Huard, K. & Lebel, H. Rhodium-catalyzed amination reaction from N-tosyloxycarbamates. Chem. Eur. J. 14, 6222–6230 (2008).
Reed, S. A., Mazzotti, A. R. & White, M. C. A catalytic, Bronsted base strategy for intermolecular allylic C–H amination. J. Am. Chem. Soc. 131, 11701–11706 (2009).
Liang, C. et al. Toward a synthetically useful stereoselective C–H amination of hydrocarbons. J. Am. Chem. Soc. 130, 343–350 (2008).
Grigg, D. R., Rigoli, J. W., Pearce, S. D. & Schomaker, J. M. Synthesis of propargylic and allenic carbamates via the C–H amination of alkynes. Org. Lett. 14, 280–283 (2012).
Thornton, A. R. & Blakey, S. B. Catalytic metallonitrene/alkyne metathesis: a powerful cascade process for the synthesis of nitrogen-containing molecules. J. Am. Chem. Soc. 130, 5020–5021 (2008).
Fleming, J. J., McReynolds, M. D. & Du Bois, J. (+)-Saxitoxin: a first and second generation stereoselective synthesis. J. Am. Chem. Soc. 129, 9964–9975 (2007).
Sharma, A. & Hartwig, J. H. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Michaudel, Q., Thevenet, D. & Baran, P. S. Intermolecular Ritter-type C–H amination of unactivated sp3 carbons. J. Am. Chem. Soc. 134, 2547–2550 (2012).
Zhong, H. A., Labinger, J. A. & Bercaw, J. E. C–H bond activation by cationic platinum(II) complexes: ligand electronic and steric effects. J. Am. Chem. Soc. 124, 1378–1399 (2002).
Meléndez, R. E. & Lubell, W. D. Synthesis and reactivity of cyclic sulfamidites and sulfamidates. Tetrahedron 59, 2581–2616 (2003).
Kang, T., Kim, Y., Lee, D., Wang, Z. & Chang, S. Iridium-catalyzed intermolecular amidation of sp3 C–H bonds: late-stage functionalization of an unactivated methyl group. J. Am. Chem. Soc. 136, 4141–4144 (2014).
Drag-Zalesinska, M. et al. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorg. Med. Chem. Lett. 19, 4814–4817 (2009).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Greenwood, N. N. & Earnshaw, A. Chemistry of the Elements 2nd edn (Elsevier, 1997).
Acknowledgements
Financial support for this work was provided by the NIH/NIGMS (GM112492). S.M.P. was a NSF Graduate Research Fellow, J.R.G is a NSF Graduate Research Fellow and a Springborn Graduate Fellow, J.P.Z. is a R.C. Fuson graduate fellow, and S.M.M. is a UIUC Summer Undergraduate Research Fellow. The authors thank Bristol-Myers-Squibb for generous support with an unrestricted ‘Freedom to Discover’ grant to M.C.W. and acknowledge J.M. Howell, J.R. Clark and C.C. Pattillo for checking our experimental procedure. The authors thank J. Bertke and D. Gray for crystallographic analysis of compound 56, L. Zhu for assistance with NMR data analysis, and D. Loudermilk for creative graphic assistance.
Author information
Authors and Affiliations
Contributions
S.M.P., J.R.G., J.P.Z., A.L.P. and S.M.M. conducted the experiments and analysed the data. S.M.P., J.R.G. and M.C.W. conceived and designed the project, analysed the data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The University of Illinois has filed a patent application on the [Mn(tBuPc)] catalyst for general C–H functionalizations. The [Mn(tBuPc)] catalyst (prod # 799688) will be offered by Aldrich through a licence from the University of Illinois.
Supplementary information
Supplementary information
Supplementary information (PDF 9159 kb)
Supplementary information
Crystallographic data for compound 56 (CIF 2960 kb)
Rights and permissions
About this article
Cite this article
Paradine, S., Griffin, J., Zhao, J. et al. A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)–H amination. Nature Chem 7, 987–994 (2015). https://doi.org/10.1038/nchem.2366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2366
This article is cited by
-
Combining visible-light induction and copper catalysis for chemo-selective nitrene transfer for late-stage amination of natural products
Communications Chemistry (2022)
-
C–H activation
Nature Reviews Methods Primers (2021)
-
Cu-catalysed intramolecular radical enantioconvergent tertiary β-C(sp3)–H amination of racemic ketones
Nature Catalysis (2020)
-
Chemoselective methylene oxidation in aromatic molecules
Nature Chemistry (2019)
-
An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds
Nature Chemistry (2019)