Abstract
Cytokinesis in many eukaryotes involves the contraction of an actomyosin-based contractile ring1,2. However, the detailed mechanism of contractile ring contraction is not fully understood. Here, we establish an experimental system to study contraction of the ring to completion in vitro. We show that the contractile ring of permeabilized fission yeast cells undergoes rapid contraction in an ATP- and myosin-II-dependent manner in the absence of other cytoplasmic constituents. Surprisingly, neither actin polymerization nor its disassembly is required for contraction of the contractile ring, although addition of exogenous actin-crosslinking proteins blocks ring contraction. Using contractile rings generated from fission yeast cytokinesis mutants, we show that not all proteins required for assembly of the ring are required for its contraction in vitro. Our work provides the beginnings of the definition of a minimal contraction-competent cytokinetic ring apparatus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Glotzer, M. The molecular requirements for cytokinesis. Science 307, 1735–1739 (2005).
Pollard, T. D. Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 22, 50–56 (2010).
Mabuchi, I. Biochemical aspects of cytokinesis. Int. Rev. Cytol. 101, 175–213 (1986).
Schroeder, T. E. in Molecules and Cell Movements (eds Inoué, S. & Stephens, R. E.) 305–334 (Raven, 1975).
Mabuchi, I., Tsukita, S., Tsukita, S. & Sawai, T. Cleavage furrow isolated from newt eggs: contraction, organization of the actin filaments, and protein components of the furrow. Proc. Natl Acad. Sci. USA 85, 5966–5970 (1988).
Robinson, D. N. & Spudich, J. A. Mechanics and regulation of cytokinesis. Curr. Opin. Cell Biol. 16, 182–188 (2004).
Pollard, T. D. & Wu, J. Q. Understanding cytokinesis: lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 11, 149–155 (2010).
Wolfe, B. A. & Gould, K. L. Split decisions: Coordinating cytokinesis in yeast. Trends Cell Biol. 15, 10–18 (2005).
Mishra, M. et al. Cylindrical cellular geometry ensures fidelity of division site placement in fission yeast. J. Cell Sci. 125, 3850–3857 (2012).
Young, B. A., Buser, C. & Drubin, D. G. Isolation and partial purification of the Saccharomyces cerevisiae cytokinetic apparatus. Cytoskeleton 67, 13–22 (2010).
Motegi, F., Arai, R. & Mabuchi, I. Identification of two type V myosins in fission yeast, one of which functions in polarized cell growth and moves rapidly in the cell. Mol. Biol. Cell 12, 1367–1380 (2001).
Pelham, R. J. & Chang, F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419, 82–86 (2002).
Wu, J. Q., Kuhn, J. R., Kovar, D. R. & Pollard, T. D. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723–734 (2003).
Arai, R. & Mabuchi, I. F-actin ring formation and the role of F-actin cables in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 115, 887–898 (2002).
Kamasaki, T., Osumi, M. & Mabuchi, I. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J. Cell Biol. 178, 765–771 (2007).
Mabuchi, I. & Okuno, M. The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 74, 251–263 (1977).
Straight, A. F. et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743–1747 (2003).
Nakamura, M., Kakuda, T., Oba, Y., Ojika, M. & Nakamura, H. Synthesis of biotinylated xestoquinone that retains inhibitory activity against Ca2+ ATPase of skeletal muscle myosin. Bioorg. Med. Chem. 11, 3077–3082 (2003).
Kitayama, C., Sugimoto, A. & Yamamoto, M. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 137, 1309–1319 (1997).
Motegi, F., Nakano, K., Kitayama, C., Yamamoto, M. & Mabuchi, I. Identification of Myo3, a second type-II myosin heavy chain in the fission yeast Schizosaccharomyces pombe. FEBS Lett. 420, 161–166 (1997).
Bezanilla, M., Forsburg, S. L. & Pollard, T. D. Identification of a second myosin-II in Schizosaccharomyces pombe: Myp2p is conditionally required for cytokinesis. Mol. Biol. Cell 8, 2693–2705 (1997).
Balasubramanian, M. et al. Isolation and characterization of new fission yeast cytokinesis mutants. Genetics 149, 1265–1275 (1998).
Lord, M. & Pollard, T. D. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol. 167, 315–325 (2004).
Wong, K. C., Naqvi, N. I., Iino, Y., Yamamoto, M. & Balasubramanian, M. K. Fission yeast Rng3p: An UCS-domain protein that mediates myosin II assembly during cytokinesis. J. Cell Sci. 113, 2421–2432 (2000).
McCollum, D., Balasubramanian, M. K., Pelcher, L. E., Hemmingsen, S. M. & Gould, K. L. Schizosaccharomyces pombe cdc4+ gene encodes a novel EF-hand protein essential for cytokinesis. J. Cell Biol. 130, 651–660 (1995).
Mendes Pinto, I., Rubinstein, B., Kucharavy, A., Unruh, J. R. & Li, R. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell 22, 1247–1260 (2012).
Nakano, K. & Mabuchi, I. Actin-depolymerizing protein Adf1 is required for formation and maintenance of the contractile ring during cytokinesis in fission yeast. Mol. Biol. Cell 17, 1933–1945 (2006).
Kovar, D. R., Kuhn, J. R., Tichy, A. L. & Pollard, T. D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875–887 (2003).
Chang, F., Drubin, D. & Nurse, P. cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137, 169–182 (1997).
Roberts-Galbraith, R. H. et al. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol. Cell 39, 86–99 (2010).
Balasubramanian, M. K., Helfman, D. M. & Hemmingsen, S. M. A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature 360, 84–87 (1992).
Stark, B. C., Sladewski, T. E., Pollard, L. W. & Lord, M. Tropomyosin and myosin-II cellular levels promote actomyosin ring assembly in fission yeast. Mol. Biol. Cell 21, 989–1000 (2010).
Eng, K., Naqvi, N. I., Wong, K. C. & Balasubramanian, M. K. Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr. Biol. 8, 611–621 (1998).
Nakano, K., Satoh, K., Morimatsu, A., Ohnuma, M. & Mabuchi, I. Interactions among a fimbrin, a capping protein, and an actin-depolymerizing factor in organization of the fission yeast actin cytoskeleton. Mol. Biol. Cell 12, 3515–3526 (2001).
Takaine, M., Numata, O. & Nakano, K. Fission yeast IQGAP arranges actin filaments into the cytokinetic contractile ring. EMBO J. 28, 3117–3131 (2009).
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 (1991).
Bähler, J. et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 (1998).
Kobori, H., Yamada, N., Taki, A. & Osumi, M. Actin is associated with the formation of the cell wall in reverting protoplasts of the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 94, 635–646 (1989).
Kamasaki, T., Arai, R., Osumi, M. & Mabuchi, I. Directionality of F-actin cables changes during the fission yeast cell cycle. Nat. Cell Biol. 7, 916–917 (2005).
Arai, R., Nakano, K. & Mabuchi, I. Subcellular localization and possible function of actin, tropomyosin and actin-related protein 3 (Arp3) in the fission yeast Schizosaccharomyces pombe. Eur. J. Cell Biol. 76, 288–295 (1998).
Fiske, C. H. & Subbarow, Y. The Colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400 (1925).
Acknowledgements
We thank D. McCollum (University of Massachusetts Medical School, USA), K. Gould (Vanderbilt University, USA), J-Q. Wu (Ohio State University, USA), V. Simanis (ISREC, Switzerland), J. Bähler (University College London, UK), T. D. Pollard (Yale University, USA), T. Toda (Cancer Research, UK), I. Hagan (Paterson Institute, UK), F. Chang (Columbia University, USA), D. Mulvihill (University of Kent, UK), P. Perez (CSIC, Spain), Y. Hiraoka (Osaka University, Japan), K. Nakano (University of Tsukuba, Japan), S. Oliferenko (TLL, Singapore), and M. Sato (Waseda University, Japan) and the Yeast Genetic Resource Center Japan for providing plasmids and strains, Y. Oba and M. Ojika (Nagoya University, Japan) for their kind gift of DXQ, Y. Toyoshima (University of Tokyo, Japan) for her kind gift of kinesin, R. Amikura for help in electron microscopy, and S. Oliferenko, S. Bulchand and D. Subramanian for critical reading of this manuscript. We thank K. Gull (Oxford University, UK) for antibodies. We thank M. Sevugan for technical assistance. This work was supported by a Japan Society for Promotion of Science (JSPS) grant-in-aid for scientific research (I.M., #22247031); JSPS research fellowships for young scientists (J.K.); NUS JSPS collaborative grant (I.M. and M.B.), Temasek Life Sciences Laboratory and Singapore Millennium Foundation (M.B. and M.M.); visiting scientist fellowship from the Gakushuin University (M.M.) and Mechanobiology Institute (M.B., R.S. and Y.H.).
Author information
Authors and Affiliations
Contributions
I.M. (in vitro activation and biochemistry) and M.K.B. (establishment of ring assembly in protoplasts and mutant studies) conceived the study, I.M., M.M., M.K.B. and J.K. designed the experiments. I.M., J.K., M.M., T.T., R.S. and Y.H. conducted the experiments. J.K., I.M. and M.M. analysed the data. M.K.B., M.M., J.K. and I.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1242 kb)
Supplementary Table 1
Supplementary Information (XLS 30 kb)
Supplementary Table 2
Supplementary Information (XLS 21 kb)
Supplementary Table 3
Supplementary Information (XLS 21 kb)
Supplementary Table 4
Supplementary Information (XLS 19 kb)
Supplementary Table 5
Supplementary Information (XLS 40 kb)
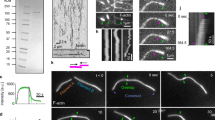
ATP drives ring contraction in vitro.
A contracting ring in the presence of 0.5 mM ATP at 25 °C corresponds to the frames shown in Fig. 2a. X-Y and Y–Z images were shown as non-tilted and 90°-tilted maximum projections of Z-stacks, respectively. (MOV 199 kb)
ATP drives ring contraction.
A contracting ring in the presence of 0.01 mM ATP at 25 °C corresponds to the frames shown in Fig. 2c. X–Y and Y–Z images were shown as non-tilted and 90°-tilted maximum projections of Z-stacks, respectively. (MOV 118 kb)
AMP-PNP does not induce ring contraction in vitro.
Experiment was done at 25 °C. The video corresponds to the frames shown in Fig. 2d. XY and Y–Z images were shown as non-tilted and 90°-tilted maximum projections of Z-stacks, respectively. (MOV 1038 kb)
The plasma membrane of Rlc1-3xGFP expressing cell ghosts stained with FM4-64 during contraction in vitro.
Experiment was done at 25 °C. The membrane did not ingress as the ring contracts. The video corresponds to the frames shown in Fig. 2g. (MOV 450 kb)
Incomplete actomyosin arcs do not show ATP-dependent contraction in vitro.
The GFP fluorescence decays the presence of 0.5 mM ATP but the actomyosin arc do not show any contraction at 25 °C. The video corresponds to the frames shown in Fig. 2j. X–Y and Y–Z images were shown as non-tilted and 90°-tilted maximum projections of Z-stacks, respectively. (MOV 329 kb)
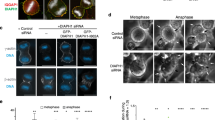
Contractile ring of myo2-E1ts undergoes slow in vitro contraction even at the permissive temperature of 25 °C.
The video corresponds to the frames shown in Fig. 3b. (MOV 105 kb)
Contractile ring of myp2 null mutant (myp2Δ) in vitro.
Experiment was done at 25 °C. The video corresponds to the frames shown in Fig. 3b. (MOV 287 kb)
Contractile ring contraction of the double mutant of myo2 and myp2 (myo2-E1ts myp2Δ) in vitro.
These rings did not show any contraction and instead fragmented and disassembled in presence of ATP at 25 °C. The video corresponds to the frames shown in Fig. 3b. (MOV 912 kb)
Addition of Jasplakinolide does not affect ring contraction in vitro.
Experiment was done at 25 °C. (MOV 548 kb)
ADF/cofilin (Adf1p) is not required for ring contraction in vitro.
Cell ghosts from adf1-1ts were held at 36 °C for 15 min before ATP addition. (MOV 534 kb)
LatA has minimal effect on ring contraction in vitro.
Experiment was done at 25 °C. (MOV 515 kb)
Formin (Cdc12p) is not required for ring contraction in vitro.
Experiment was done at 36 °C. Cell ghosts from cdc12-112ts were held at 36 °C for 15 min before ATP addition. (MOV 204 kb)
F-BAR protein (Cdc15p) is not required for ring contraction in vitro.
Experiment was done at 36 °C. Cell ghosts from cdc15-140ts were held at 36 °C for 15 minutes before ATP addition. (MOV 571 kb)
Tropomyosin (Cdc8p) is required for integrity and contraction of ring in vitro.
Cell ghosts from cdc8-110ts were held at 36 °C for 15 min before ATP addition. The video corresponds to the frames shown in Fig. 5a. (MOV 105 kb)
Addition of 1 μM purified N-terminal region of IQGAP (Rng2Ns) completely blocks ring contraction in vitro.
Experiment was done at 25 °C. The video corresponds to the frames shown in Fig. 5b. (MOV 253 kb)
Addition of 0.9 μM purified fimbrin (Fim1p) completely blocks ring contraction in vitro.
Experiment was done at 25 °C. The video corresponds to the frames shown in Fig. 5c. (MOV 212 kb)
Rights and permissions
About this article
Cite this article
Mishra, M., Kashiwazaki, J., Takagi, T. et al. In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics. Nat Cell Biol 15, 853–859 (2013). https://doi.org/10.1038/ncb2781
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2781
This article is cited by
-
Anillin propels myosin-independent constriction of actin rings
Nature Communications (2021)
-
Synthetic cell division via membrane-transforming molecular assemblies
BMC Biology (2019)
-
Coupled circumferential and axial tension driven by actin and myosin influences in vivo axon diameter
Scientific Reports (2017)
-
Model organism databases: essential resources that need the support of both funders and users
BMC Biology (2016)
-
Still and rotating myosin clusters determine cytokinetic ring constriction
Nature Communications (2016)