Abstract
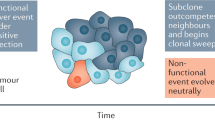
Cancer is often regarded as a process of asexual evolution driven by genomic and genetic instability. Mutation, selection and adaptation are by convention thought to occur primarily within, and to a lesser degree outside, the primary tumour. However, disseminated cancer cells that remain after 'curative' surgery exhibit extreme genomic heterogeneity before the manifestation of metastasis. This heterogeneity is later reduced by selected clonal expansion, suggesting that the disseminated cells had yet to acquire key traits of fully malignant cells. Abrogation of the cells' progression outside the primary tumour implies new challenges and opportunities for diagnosis and adjuvant therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Douillard, J. Y. et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 28, 4697–4705 (2010).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Alberts, S. R. et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. J. Am. Med. Assoc. 307, 1383–1393 (2012).
Goss, G. D. et al. A phase III randomized, double-blind, placebo-controlled trial of epidermal growth factor receptor inhibitor gefitinib in completely resected stage IB-IIIA non-small cell lung cancer (NSCLC): NCIC CTG BR.19. J. Clin. Oncol. 28 (suppl.), abstr. LBA7005 (2010).
Polzer, B. & Klein, C. A. Metastasis awakening: the challenges of targeting minimal residual cancer. Nature Med. 19, 274–275 (2013).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012). This review summarizes current evolutionary concepts of cancer.
Nguyen, D. X., Bos, P. D. & Massague, J. Metastasis: from dissemination to organ-specific colonization. Nature Rev. Cancer 9, 274–284 (2009).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Kang, Y. & Pantel, K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell 23, 573–581 (2013).
Hafner, C. et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc. Natl Acad. Sci. USA 104, 13450–13454 (2007).
Hafner, C. et al. Multiple oncogenic mutations and clonal relationship in spatially distinct benign human epidermal tumors. Proc. Natl Acad. Sci. USA 107, 20780–20785 (2010). References 14 and 15 provide insight into the genetics and biology of benign skin tumours.
Naski, M. C., Wang, Q., Xu, J. & Ornitz, D. M. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nature Genet. 13, 233–237 (1996).
Allred, D. C. et al. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin. Cancer Res. 14, 370–378, (2008).
Boecker, W. et al. Ductal epithelial proliferations of the breast: a biological continuum? Comparative genomic hybridization and high-molecular-weight cytokeratin expression patterns. J. Pathol. 195, 415–421 (2001).
Chin, K. et al. In situ analyses of genome instability in breast cancer. Nature Genet. 36, 984–988 (2004). This paper suggests that telomere crisis could result in an evolutionary shift at the transition from benign to malignant tumours.
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Winawer, S. J. et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130, 1872–1885 (2006).
Carr, N. J., Mahajan, H., Tan, K. L., Hawkins, N. J. & Ward, R. L. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J. Clin. Pathol. 62, 516–518 (2009).
Bettington, M. et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology 62, 367–386 (2013).
Toyota, M. et al. CpG island methylator phenotype in colorectal cancer. Proc. Natl Acad. Sci. USA 96, 8681–8686 (1999).
Neuville, A. et al. Histologic characteristics of non-microsatellite-instable colon adenomas correlate with distinct molecular patterns. Hum. Pathol. 42, 244–253 (2011).
Bardi, G. et al. Cytogenetic comparisons of synchronous carcinomas and polyps in patients with colorectal cancer. Br. J. Cancer 76, 765–769 (1997).
Heng, H. H. et al. Evolutionary mechanisms and diversity in cancer. Adv. Cancer Res. 112, 217–253 (2011).
Mitelman, F., Johansson, B. & Mertens, F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer http://cgap.nci.nih.gov/Chromosomes/Mitelman (NCI, 2013) .
Teixeira, M. R. & Heim, S. Cytogenetic analysis of tumor clonality. Adv. Cancer Res. 112, 127–149 (2011).
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nature Genet. 38, 468–473 (2006). This paper established that measures of genetic diversity are a novel form of biomarker.
Park, S. Y., Gonen, M., Kim, H. J., Michor, F. & Polyak, K. Cellular and genetic diversity in the progression of in situ human breast carcinomas to an invasive phenotype. J. Clin. Invest. 120, 636–644 (2010).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Engel, J. et al. The process of metastasisation for breast cancer. Eur. J. Cancer 39, 1794–1806 (2003).
Klein, C. A. & Holzel, D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle 5, 1788–1798 (2006).
Klein, C. A. Parallel progression of primary tumours and metastases. Nature Rev. Cancer 9, 302–312 (2009).
Banys, M. et al. Hematogenous and lymphatic tumor cell dissemination may be detected in patients diagnosed with ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 131, 801–808 (2012).
Hüsemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). This paper established that metastatic dissemination might occur shortly after malignant transformation in transgenic mouse models and patients.
Sänger, N. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Klein, C. A. et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet 360, 683–689 (2002).
Schardt, J. A. et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell 8, 227–239 (2005).
Schmidt-Kittler, O. et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl Acad. Sci. USA 100, 7737–7742 (2003).
Stoecklein, N. H. et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell 13, 441–453 (2008).
Weckermann, D. et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J. Clin. Oncol. 27, 1549–1556 (2009). References 39 to 43 provide genomic data for single DCCs.
Rasnick, D. Aneuploidy theory explains tumor formation, the absence of immune surveillance, and the failure of chemotherapy. Cancer Genet. Cytogenet. 136, 66–72 (2002).
Magbanua, M. J. et al. Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res. 73, 30–40 (2013).
Magbanua, M. J. et al. Isolation and genomic analysis of circulating tumor cells from castration resistant metastatic prostate cancer. BMC Cancer 12, 78 (2012).
Heitzer, E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013).
Baudis, M. & Cleary, M. L. Progenetix.net: an online repository for molecular cytogenetic aberration data. Bioinformatics 12, 1228–1229 (2001).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Theissen, G. Saltational evolution: hopeful monsters are here to stay. Theory Biosci. 128, 43–51 (2009).
Tang, Y. C. & Amon, A. Gene copy-number alterations: a cost-benefit analysis. Cell 152, 394–405 (2013).
Schlimok, G. et al. Micrometastatic cancer cells in bone marrow: in vitro detection with anti-cytokeratin and in vivo labeling with anti-17–1A monoclonal antibodies. Proc. Natl Acad. Sci. USA 84, 8672–8676 (1987). This paper may be considered as laying the foundation of modern DCC and CTC research by establishing the fundamental concept and the most relevant detection markers.
Cauffman, G., De Rycke, M., Sermon, K., Liebaers, I. & Van de Velde, H. Markers that define stemness in ESC are unable to identify the totipotent cells in human preimplantation embryos. Hum. Reprod. 24, 63–70 (2009).
Lu, T. Y. et al. Epithelial cell adhesion molecule regulation is associated with the maintenance of the undifferentiated phenotype of human embryonic stem cells. J. Biol. Chem. 285, 8719–8732 (2010).
Ng, V. Y., Ang, S. N., Chan, J. X. & Choo, A. B. Characterization of epithelial cell adhesion molecule as a surface marker on undifferentiated human embryonic stem cells. Stem Cells 28, 29–35 (2010).
Moll, R., Franke, W. W., Schiller, D. L., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Göttlinger, H., Johnson, J. & Riethmuller, G. Biochemical and epitope analysis of the 17–1A membrane antigen. Hybridoma 5, S29–S37 (1986).
Gudjonsson, T. et al. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693–706 (2002).
Brabletz, T. et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA 98, 10356–10361 (2001).
Gires, O., Klein, C. A. & Baeuerle, P. A. On the abundance of EpCAM on cancer stem cells. Nature Rev. Cancer 9, 143 (2009).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Rev. Cancer 8, 755–768 (2008).
Riethdorf, S., Wikman, H. & Pantel, K. Biological relevance of disseminated tumor cells in cancer patients. Int. J. Cancer 123, 1991–2006 (2008).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011). This paper provides the first evidence that DCCs may compete for niches with autochthonous cells.
Engel, J., Emeny, R. T. & Holzel, D. Positive lymph nodes do not metastasize. Cancer Metastasis Rev. 31, 235–246 (2012).
Fisher, B. et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N. Engl. J. Med. 347, 567–575 (2002).
Kitchener, H., Swart, A. M., Qian, Q., Amos, C. & Parmar, M. K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 373, 125–136 (2009).
Rudenstam, C. M. et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: first results of International Breast Cancer Study Group. Trial 10–93. J. Clin Oncol. 24, 337–344 (2006).
Veronesi, U., Marubini, E., Mariani, L., Valagussa, P. & Zucali, R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur. J. Cancer 35, 1320–1325 (1999).
Stoecklein, N. H. & Klein, C. A. Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int. J. Cancer 126, 589–598 (2010).
Kloosterman, W. P. et al. Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer. Genome Biol. 12, R103 (2011).
Miranda, E. et al. Genetic and epigenetic alterations in primary colorectal cancers and related lymph node and liver metastases. Cancer 119, 266–276 (2013).
Shah, S. P. et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461, 809–813 (2009).
Vermaat, J. S. et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin. Cancer Res. 18, 688–699 (2012).
Campbell, P. J. et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113 (2010).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Castellarin, M. et al. Clonal evolution of high-grade serous ovarian carcinoma from primary to recurrent disease. J. Pathol. 229, 515–524 (2013).
Ding, L. et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Klein, C. A. Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49 (2011).
Weedon-Fekjaer, H., Lindqvist, B. H., Vatten, L. J., Aalen, O. O. & Tretli, S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 10, R41 (2008). The most extensive study on growth rates of breast cancer.
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Kang, Y. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Minn, A. J. et al. Genes that mediate breast cancer metastasis to lung. Nature 436, 518–524 (2005).
Berenblum, I. & Shubik, P. The role of croton oil applications, associated with a single painting of a carcinogen, in tumour induction of the mouse's skin. Br. J. Cancer 1, 379–382 (1947).
Van Duuren, B. L., Sivak, A., Katz, C., Seidman, I. & Melchionne, S. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 35, 502–505 (1975).
Aaronson, S. A. & Todaro, G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science 162, 1024–1026 (1968).
Lee, L. W., Tsao, M. S., Grisham, J. W. & Smith, G. J. Emergence of neoplastic transformants spontaneously or after exposure to N-methyl-N9-nitro-N-nitrosoguanidine in populations of rat liver epithelial cells cultured under selective and nonselective conditions. Am. J. Pathol. 135, 63–71 (1989).
Rubin, H. Cell–cell contact interactions conditionally determine suppression and selection of the neoplastic phenotype. Proc. Natl Acad. Sci. USA 105, 6215–6221 (2008).
Johnston, L. A. Competitive interactions between cells: death, growth, and geography. Science 324, 1679–1682 (2009).
Levayer, R. & Moreno, E. Mechanisms of cell competition: themes and variations. J. Cell Biol. 200, 689–698 (2013).
Moreno, E. Is cell competition relevant to cancer? Nature Rev. Cancer 8, 141–147 (2008). References 91 and 92 summarize the evidence for a role for cell competition in cancer.
Mueller, M. M. Inflammation in epithelial skin tumours: old stories and new ideas. Eur. J. Cancer 42, 735–744 (2006).
O'Reilly, M. S. et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328 (1994).
Ruggiero, R. A. et al. Tyrosine isomers mediate the classical phenomenon of concomitant tumor resistance. Cancer Res. 71, 7113–7124 (2011).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
McAllister, S. S. et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 133, 994–1005 (2008).
Hiratsuka, S. et al. The S100A8-serum amyloid A3–TLR4 paracrine cascade establishes a pre-metastatic phase. Nature Cell Biol. 10, 1349–1355 (2008).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Med. 18, 883–891 (2012). Reference 96 to 99 provide insight into how primary tumour-induced systemic alterations are linked to metastatic success of DCCs.
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nature Cell Biol. 15, 807–817 (2013).
Klein, C. A. & Stoecklein, N. H. Lessons from an aggressive cancer: evolutionary dynamics in esophageal carcinoma. Cancer Res. 69, 5285–5288 (2009).
Tang, Y. C., Williams, B. R., Siegel, J. J. & Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell 144, 499–512 (2011).
Steeg, P. S. The right trials. Nature 485, S58–S59 (2012).
Hölzel, D., Eckel, R., Emeny, R. T. & Engel, J. Distant metastases do not metastasize. Cancer Metastasis Rev. 29, 737–750 (2010).
Acknowledgements
I am indebted to T. Perry for his critical reading of the manuscript and his invaluable suggestions to improve it. I am also grateful to S. Pausch for her help with the figures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Klein, C. Selection and adaptation during metastatic cancer progression. Nature 501, 365–372 (2013). https://doi.org/10.1038/nature12628
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12628
This article is cited by
-
Growth exponents reflect evolutionary processes and treatment response in brain metastases
npj Systems Biology and Applications (2023)
-
Intracellular and extracellular factors of colorectal cancer liver metastasis: a pivotal perplex to be fully elucidated
Cancer Cell International (2022)
-
Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression
British Journal of Cancer (2021)
-
Long non-coding RNA NR2F1-AS1 induces breast cancer lung metastatic dormancy by regulating NR2F1 and ΔNp63
Nature Communications (2021)
-
How is cancer complex?
European Journal for Philosophy of Science (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.