Abstract
Objective:
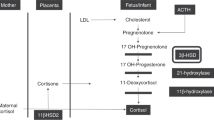
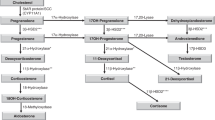
Screening for congenital adrenal hyperplasia (CAH) caused by 21-α-hydroxylase deficiency is challenging because factors such as prematurity and stress increase intermediate steroid metabolite levels in newborn infants. The objective of this study was to explore the use of the 17-α-hydroxyprogesterone (17-OHP)/11-deoxycortisol ratio as an adjunct measure in the follow-up evaluation of infants with presumptive positive newborn screens for CAH to distinguish between infants with no disorder and those with CAH.
Study Design:
This was a retrospective cohort study of infants with presumptive positive newborn screens for CAH. The precursor-to-product ratio of 17-OHP/11-deoxycortisol was compared between infants with no disorder (n=47) and infants with CAH (n=5).
Results:
The CAH infants had higher 17-OHP/11-deoxycortisol ratios than infants with no disorder: 26 (18 to 58) and 1.05 (0.69 to 1.46), respectively (P<0.05). Among infants with no disorder, higher levels of serum 17-OHP did not reflect higher ratios, indicating sufficient enzyme activity.
Conclusion:
The results suggest that a low 17-OHP/11-deoxycortisol ratio represents 21-α-hydroxylase sufficiency among presumptive positives in newborn screening of CAH.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
CDPH. California Newborn Screening Program. 2015 4/15/2015 (cited 27 October 2015, webpage). Available from https://www.cdph.ca.gov/programs/nbs/Pages/NBSProgrOVforProviders.aspx.
Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95 (9): 4133–4160.
Hayashi G, Faure C, Brondi MF, Vallejos C, Soares D, Oliveira É et al. Weight-adjusted neonatal 17OH-progesterone cutoff levels improve the efficiency of newborn screening for congenital adrenal hyperplasia. Arq Bras Endocrinol Metabol 2011; 55: 632–637.
Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA . Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res 2002; 52 (3): 405–410.
Nordenstrom A, Wedell A, Hagenfeldt L, Marcus C, Larsson A . Neonatal screening for congenital adrenal hyperplasia: 17-hydroxyprogesterone levels and CYP21 genotypes in preterm infants. Pediatrics 2001; 108 (4): E68.
Ersch J, Beinder E, Stallmach T, Bucher HU, Torresani T . 17-Hydroxyprogesterone in premature infants as a marker of intrauterine stress. J Perinat Med 2008; 36 (2): 157–160.
Fingerhut R . False positive rate in newborn screening for congenital adrenal hyperplasia (CAH)-ether extraction reveals two distinct reasons for elevated 17alpha-hydroxyprogesterone (17-OHP) values. Steroids 2009; 74 (8): 662–665.
Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA . Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab 1994; 78 (2): 266–270.
Sarafoglou K, Banks K, Gaviglio A, Hietala A, McCann M, Thomas W . Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics 2012; 130 (5): e1261–e1268.
Hicks RA, Yee JK, Mao CS, Graham S, Kharrazi M, Lorey F et al. Precursor-to-product ratios reflect biochemical phenotype in congenital adrenal hyperplasia. Metabolomics 2014; 10 (1): 123–131.
van der Kamp HJ, Oudshoorn CG, Elvers BH, van Baarle M, Otten BJ, Wit JM et al. Cutoff levels of 17-alpha-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J Clin Endocrinol Metab 2005; 90 (7): 3904–3907.
White PC . Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol 2009; 5 (9): 490–498.
Trapp CM, Speiser PW, Oberfield SE . Congenital adrenal hyperplasia: an update in children. Curr Opin Endocrinol Diabetes Obes 2011; 18 (3): 166–170.
Huynh T, McGown I, Cowley D, Nyunt O, Leong GM, Harris M et al. The clinical and biochemical spectrum of congenital adrenal hyperplasia secondary to 21-hydroxylase deficiency. Clin Biochem Rev 2009; 30 (2): 75–86.
Doerr HG, Sippell WG, Versmold HT, Bidlingmaier F, Knorr D . Plasma mineralocorticoids, glucocorticoids, and progestins in premature infants: longitudinal study during the first week of life. Pediatr Res 1988; 23 (5): 525–529.
Hicks RA, Fereira BF, Mao CS, Yee JK, Lee WP . The use of 17-α-hydroxyprogesterone to 11-deoxycortisol ratio in newborn screening of congenital adrenal hyperplasia. J Invest Med 2012; 60 (1): 222.
Fisher DA, Salameh W, Furlanetto RW . The Quest Diagnostics Manual: Endocrinology, Fourth Edition. Quest Diagnostics: San Juan Capistrano, 2007.
Acknowledgements
Special thanks to Barbara Ferreira, GCN Project Director Area Service Center, and the staff at the Newborn Screening Program at Harbor-UCLA Medical Center. We appreciate the advice from the University of California, Los Angeles, Clinical Translational Science Institute (UL1TR000124) in biostatistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tieh, P., Yee, J., Hicks, R. et al. Utility of a precursor-to-product ratio in the evaluation of presumptive positives in newborn screening of congenital adrenal hyperplasia. J Perinatol 37, 283–287 (2017). https://doi.org/10.1038/jp.2016.223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.223