Abstract
Circadian blood pressure variability and melatonin secretion are both regulated by the biological clock. Several clinical trials have suggested that oral administration of exogenous melatonin lowers blood pressure at night, although it remains unclear whether melatonin secretion, which is considerably lower than pharmacological melatonin levels, is associated with nighttime blood pressure. In this cross-sectional study, we measured overnight urinary melatonin excretion, which is an index of melatonin secreted, along with ambulatory blood pressure. Of 863 participants (mean age, 72.1 years), 386 participants received some form of antihypertensive drug treatment. With a quartile increase in urinary melatonin excretion, nighttime systolic blood pressure significantly decreased in the untreated group (P-value for trend=0.01), whereas neither association was observed in the treated group (P-value for trend=0.87). Among the untreated group, multivariate linear regression models revealed that higher log-transformed urinary melatonin excretion was significantly associated with decreased nighttime systolic blood pressure, independently of age, gender, body mass index, current smoking status, diabetes, daytime physical activity, duration in bed (scotoperiod) and day length (photoperiod) (regression coefficient: −2.21; 95% confidence interval: −4.38 to −0.05, P=0.045). This association suggests that an increase in the urinary melatonin excretion from 4.2 to 10.5 μg (25th to 75th percentile) is associated with a 2.0 mm Hg decrease in nighttime systolic blood pressure. In conclusion, melatonin secretion is significantly and inversely associated with nighttime blood pressure in a general elderly population without antihypertensive drug treatment. This association was not observed in treated elderly individuals.
Similar content being viewed by others
Introduction
Epidemiological studies have demonstrated that nighttime blood pressure (BP) is a stronger predictor of cardiovascular diseases and mortality than nocturnal BP dipping and daytime BP.1, 2, 3 Circadian BP variability is regulated by the suprachiasmatic nucleus that is located in the hypothalamus, which contains the master biological clock.4
Melatonin is a pineal gland hormone under the control of the suprachiasmatic nucleus. Secretion of melatonin follows a circadian rhythm, with almost all production at night.5 Melatonin receptors have been found in a majority of central and peripheral tissues, including endothelial cells.6, 7 Melatonin increases cytosolic Ca2+ and levels of nitric oxide in endothelial cells and reduces serum norepinephrine levels, resulting in the enhancement of vasodilation.8, 9, 10 Thus, melatonin plays a key role in nighttime BP regulation, and several clinical trials have suggested that oral administration of exogenous melatonin lowers BP at night.11, 12 However, the long-term efficacy of exogenous melatonin on BP reduction and the adverse effects of pharmacologic melatonin levels have not yet been determined.
With regard to endogenous melatonin levels that are considerably lower than pharmacological melatonin levels, several lines of evidence in humans suggest that melatonin secretion is associated with circadian BP regulation.13, 14, 15 Although melatonin secretion has large individual variation that is partially explained by lifestyle factors including light exposure, and that this variation may be associated with nighttime BP levels,16, 17, 18, 19 it remains unclear whether melatonin secretion is associated with nighttime BP. Moreover, the magnitudes of these potential associations have also not been determined.
To determine the association of melatonin secretion with nighttime BP, we conducted a cross-sectional study comprising 863 elderly individuals. Urinary 6-sulfatoxymelatonin excretion (UME) was used as an index of melatonin secretion because of the excretion of 6-sulfatoxymelatonin, the major metabolite of melatonin, which is closely correlated with the amount of melatonin secreted.20
Methods
Participants and study protocol
Between September 2010 and March 2013, we recruited participants aged ⩾60 years with the cooperation of local residents’ associations and elderly residents’ clubs, and a total of 880 elderly subjects voluntarily enrolled in a study titled ‘Housing Environments and Health Investigation among Japanese Older People in Nara, Kansai Region: a prospective community-based cohort (HEIJO-KYO) study’. Of these, 863 home-dwelling participants met the inclusion criteria of completed UME measurement records. All the participants provided written informed consent, and the study protocol was approved by the medical ethics committee of Nara Medical University.
The protocols were described in our previously reported study.15 In brief, we collected demographic and medical information, including the information of antihypertensive drug treatment by calcium channel blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, beta-blockers, alpha-blockers or diuretics, using a standardized questionnaire and personal interview. Then, we initiated 48-h measurements of ambulatory BP (ABP) and instructed the participants to collect their urine the following night and maintain a standardized sleep diary by making a note of the time they went to bed and the period of time they spent in bed.
UME assessment
The urine collection protocol involved discarding the last void at bedtime and collecting each subsequent void until the first morning void. Samples were stored in dark bottles with a cold pack, the total volume was measured and then the samples were stored at −20 °C until the assay. Urinary 6-sulfatoxymelatonin concentrations were measured using a highly sensitive Enzyme-Linked Immunosorbent Assay Kit (RE54031; IBL International, Hamburg, Germany) with a lower 6-sulfatoxymelatonin detection limit of 1.0 ng ml−1. When a sample had 6-sulfatoxymelatonin levels below the detection limit, we substituted the detection limit value. UME was calculated as follows: UME (μg)=6-sulfatoxymelatonin concentration (μg ml−1) × total overnight urine volume (ml). UME data were considered missing if the urine was not collected according to the protocol. The reproducibility of UME in the initial 188 participants was assessed by an additional collection of urine samples approximately 4 months later. The intraclass correlation coefficient between the two UME levels was 0.66 (95% confidence interval (CI), 0.57–0.73).
ABP monitoring
ABP monitoring was performed using a validated ambulatory recorder (TM-2430; A&D, Tokyo, Japan) and cuff on the non-dominant arm. BP was measured at 30-min intervals for 48 h. Nighttime was defined as the period in bed based on sleep diaries. BP measured <10 times during daytime or <5 times during nighttime, as the result of errors, were excluded from analyses. The mean values of 2 days data in nighttime systolic BP (SBP) and diastolic BP (DBP) were used as parameters for nighttime BP.
Other measurements
Body mass index (BMI) was calculated as weight (kg) per height (m2). Current smoking status was evaluated using a questionnaire. Venous blood samples were analyzed to determine glycated hemoglobin, fasting plasma glucose and creatinine concentrations. Diabetes mellitus was diagnosed based on the medical history, current antidiabetic treatment or when fasting plasma glucose was ⩾7.0 mmol l−1 and glycated hemoglobin level was ⩾6.5% of the National Glycohemoglobin Standardization Program value. The estimated glomerular filtration rate was calculated according to the Japanese Society of Nephrology–Chronic Kidney Disease Practice Guide.21 Duration in bed for the scotoperiod was obtained from the individual’s sleep diary. Day length for the photoperiod in Nara (latitude: 34°N) from sunrise to sunset on measurement days was obtained from the National Astronomical Observatory of Japan website.22 Physical activity was recorded at 1-min intervals for two consecutive days using an actigraph (Actiwatch 2; Respironics, Murrysville, PA, USA) on the non-dominant wrist. Daytime physical activity was determined using Actiware version 5.5 (Respironics) as the average value of activity counts during the out-of-bed period.
Statistical analyses
Variables with a normal distribution were reported as means±s.d., whereas variables with asymmetric distribution were reported as medians and interquartile ranges. Mean and median were compared between the groups with and without antihypertensive drug treatment using the unpaired t-test and Mann–Whitney U-test, respectively. The χ2 test was performed for comparison of categorical data. For data regarding ABP monitoring, duration in bed, day length and physical activity, the average of two consecutive days were used for further analyses. UME values were naturally log-transformed for analysis because of a skewed data distribution. Trends in the associations of quartiles of UME with nighttime SBP and DBP were evaluated using the Jonckheere–Terpstra test for trends.23 Univariate linear regression models in the untreated group included nighttime SBP as dependent variables, and age, gender, BMI, current smoking status, diabetes, daytime physical activity, UME, duration in bed (scotoperiod) and day length (photoperiod) as independent variables. These independent variables include light/dark conditions that affect melatonin secretion, namely photoperiod and scotoperiod, as well as established risk factors for increased nighttime BP. They were simultaneously adjusted in multivariate models. In addition, the mean log-transformed UME adjusted for age and duration in bed was compared between the groups with and without various antihypertensive drug treatments using analysis of covariance. Statistical analyses were performed using SPSS version 19.0 for Windows (IBM SPSS, Chicago, IL, USA), and a two-sided P-value <0.05 was considered statistically significant.
Results
Of the 863 participants (mean age, 72.1 years), 386 participants received some form of antihypertensive drug treatment (Table 1). The mean age and BMI were significantly higher in the treated group than those in the untreated group (both P<0.001). Diabetes was significantly more frequently observed in the treated group than in the untreated group (P=0.006). The median UME and mean daytime physical activity were significantly lower and the mean duration in bed was significantly longer in the treated group than in the untreated group (P=0.011, P<0.001 and P=0.007, respectively). None of the participants consumed any drug or supplement containing melatonin, and two participants had an estimated glomerular filtration rate value <30 ml min−1 per 1.73 m2 (mean, 72.0±14.9 ml min−1 per 1.73 m2).
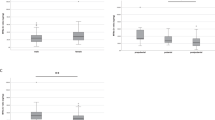
With a quartile increase in UME, mean nighttime SBP significantly decreased in the untreated group (Q1, 117.0±17.2 mm Hg; Q2, 115.7±15.6 mm Hg; Q3, 114.3±16.6 mm Hg; Q4, 112.6±17.9 mm Hg; P-value for trend=0.01; Figure 1a), whereas this change did not occur in the treated group (Figure 1b). Mean nighttime DBP did not significantly change with a quartile increase in UME (Figures 1c and d).
Differential associations of quartiles of urinary 6-sulfatoxymelatonin excretion with nighttime systolic blood pressure (SBP) (a and b) and nighttime diastolic blood pressure (DBP) (c and d) between the untreated and treated groups. Solid and error bars indicate the means and standard deviations, respectively. The P-value is shown for the trend using the Jonckheere–Terpstra test.23
Among 475 untreated participants (two missing), univariate linear regression analyses showed significant associations between nighttime SBP and age, gender, current smoking status, diabetes, daytime physical activity, log-transformed UME, duration in bed and day length (Table 2). Log-transformed UME was significantly and consistently associated with nighttime SBP both on the first and second nights (regression coefficient, −2.84; 95% CI, −5.13 to −0.55; P=0.015; regression coefficient, −2.89; 95% CI, −5.19 to −0.59; P=0.014; respectively). The multivariate model indicated that higher log-transformed UME was significantly associated with decreased nighttime SBP, independently of the covariates (regression coefficient, −2.21; 95% CI, −4.38 to −0.05; P=0.045).
The dose–response slopes (Figure 2), drawn by substituting the mean for age, BMI, daytime physical activity and duration in bed; the proportion for gender, current smoking status and diabetes; and the mean categorical number for day length to the multivariate regression formulas in Table 2 indicated that an increase in the UME from 4.2 to 10.5 μg (25th to 75th percentile) was associated with a 2.0 mm Hg decrease (95% CI, 0.04–4.0 mm Hg) in nighttime SBP (116.0–113.9 mm Hg).
With regard to the associations between UME and daytime BP, with a quartile increase in UME, daytime DBP significantly increased in the untreated group (P-value for trend=0.03). Daytime SBP in the untreated group and daytime SBP and DBP in the treated group did not significantly change with a quartile increase in UME (P-value for trend=0.69, 0.82 and 0.49; respectively). The association between log-transformed UME and daytime DBP was not significant in the multivariate model including the former covariates (regression coefficient, 0.323; 95% CI, −0.756 to 1.403; P=0.56).
Discussion
We demonstrated that higher levels of UME are significantly associated with decreased nighttime SBP in a general elderly population without antihypertensive drug treatment. The dose–response slope indicates that an increase in UME from 4.2 to 10.5 μg (25th to 75th percentile) is associated with a 2.0 mm Hg decrease (95% CI, 0.04–4.0 mm Hg) in nighttime SBP (116.0–113.9 mm Hg). This association of melatonin secretion with nighttime BP was not observed in elderly individuals treated with antihypertensive drugs. In addition, there were no significant associations between melatonin secretion and daytime BP after adjustment for potential confounding factors. To the best of our knowledge, this is the first report detailing the magnitude of the association between melatonin secretion and nighttime BP.
Previous clinical trials using oral melatonin indicated the potential effects of melatonin on both nighttime SBP and DBP. In the studies, oral melatonin (2.5 and 2 mg controlled release) significantly decreased nighttime SBP by 5.6 and 6.0 mm Hg and DBP by 3.9 and 3.0 mm Hg, respectively.11, 12 However, in the present study, UME was significantly associated with nighttime SBP rather than nighttime DBP. In experimental studies, melatonin increases cytosolic Ca2+ and levels of nitric oxide in endothelial cells, resulting in the enhancement of vasodilation.8, 9 Melatonin naturally relates to good sleep and aligned circadian rhythmicity, which are associated with reduced serum norepinephrine levels.5, 24, 25 Melatonin also acts as a highly effective antioxidant by direct and indirect free radical-scavenging actions both at physiological and pharmacological serum levels and may relate to atherosclerosis.26 Advanced atherosclerosis reduces arterial elasticity and may be more closely associated with SBP than DBP in elderly individuals, typically found in isolated systolic hypertension.27 Therefore, our result that UME is associated with nighttime SBP rather than nighttime DBP may suggest possible association between melatonin secretion and atherosclerosis. Further human research is required to better understand the association between melatonin secretion and atherosclerosis.
In contrast with the untreated group, we did not observe significant association between melatonin secretion and nighttime BP in the treated group. Some potential confounders underlying the association between melatonin secretion and nighttime BP could be considered in the treated group. Antihypertensive drug treatment may attenuate this association by reducing nighttime BP. Most participants in the treated group took long-acting antihypertensive drugs, and a considerable number of participants (n=87, data not shown) took their antihypertensive drugs in the evening or before bedtime to reduce nighttime or morning BP. Thus, current standard antihypertensive therapy, including chronotherapy, may affect nighttime BP as well as daytime BP.28 Moreover, antihypertensive agents may affect melatonin secretion. Synthesis and release of melatonin are stimulated by norepinephrine via β-adrenergic receptors, and β-blockers may block sympathetic signaling to the pineal gland, probably resulting in the suppression of melatonin secretion.5 Results of several studies have demonstrated an association between β-blockers and decreased melatonin secretion.29, 30, 31
The present study has some strengths and limitations. The strengths include simultaneously measured UME and ABP among the participants with and without antihypertensive drug treatment and analysis of these parameters treated as continuous valuables. Previous studies13, 14, 15 were limited to categorical analysis for nocturnal BP dipping (that is, dipper vs. non-dipper) with the use of arbitrary thresholds, relatively small sample sizes and the confounding effect of antihypertensive drugs. A limitation in the present study was the lack of data related to sleep quality, including sleep-disordered breathing, which is a potential confounder. However, when including sleep quality as a potential confounder, the associations of melatonin secretion with nighttime BP may be underestimated because sleep quality could be a mediator underlying the association between melatonin secretion and nighttime BP. Moreover, the association between melatonin secretion and sleep-disordered breathing was not consistent in previous studies.32, 33 Another limitation was non-random sampling because participants were recruited with the cooperation of local-resident associations and elderly-resident clubs, possibly leading to selection bias (participation rate unknown). However, the generalizability of our findings may be acceptable because some basic data (for example, BMI and estimated glomerular filtration rate) were consistent with those of the National Health and Nutrition Survey in Japan in 2010.34
In conclusion, the present study demonstrated the independent association of melatonin levels with decreased nighttime BP in a general elderly population without antihypertensive drug treatment. In contrast, this association of melatonin secretion with nighttime BP was not observed in treated elderly individuals.
References
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA . Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370: 1219–1229.
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA . Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008; 51: 55–61.
Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G . Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783.
Paschos GK, FitzGerald GA . Circadian clocks and vascular function. Circ Res 2010; 106: 8337–8341.
Brzezinski A . Melatonin in humans. N Engl J Med 1997; 336: 186–195.
Drew JE, Barrett P, Mercer JG, Moar KM, Canet E, Delagrange P, Morgan PJ . Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J Neuroendocrinol 2001; 13: 453–458.
Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W . The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res 2003; 35: 40–44.
Pogan L, Bissonnette P, Parent L, Sauvé R . The effects of melatonin on Ca2+ homeostasis in endothelial cells. J Pineal Res 2002; 33: 37–47.
Anwar MM, Meki AR, Rahma HH . Inhibitory effects of melatonin on vascular reactivity: possible role of vasoactive mediators. Comp Biochem Physiol C 2001; 130: 357–367.
Arangino S, Cagnacci A, Angiolucci M, Vacca AM, Longu G, Volpe A, Melis GB . Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol 1999; 83: 1417–1419.
Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM . Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004; 43: 192–197.
Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N . Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med 2006; 119: 898–902.
Zeman M, Dulková K, Bada V, Herichová I . Plasma melatonin concentrations in hypertensive patients with the dipping and non-dipping blood pressure profile. Life Sci 2005; 76: 1795–1803.
Jonas M, Garfinkel D, Zisapel N, Laudon M, Grossman E . Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press 2003; 12: 19–24.
Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N . Nocturnal urinary melatonin excretion is associated with non-dipper pattern in elderly hypertensives. Hypertens Res 2013; 36: 736–740.
Burgess HJ, Fogg LF . Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE 2008; 3: e3055.
Schernhammer ES, Kroenke CH, Dowsett M, Folkerd E, Hankinson SE . Urinary 6-sulfatoxymelatonin levels and their correlations with lifestyle factors and steroid hormone levels. J Pineal Res 2006; 40: 116–124.
Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C . Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 2000; 526: 695–702.
Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, Ikada Y, Kurumatani N . Positive effect of daylight exposure on nocturnal urinary melatonin excretion in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab 2012; 97: 4166–4173.
Baskett JJ, Cockrem JF, Antunovich TA . Sulphatoxymelatonin excretion in older people: relationship to plasma melatonin and renal function. J Pineal Res 1998; 24: 58–61.
Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H, Nose T . The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 2005; 69: 1264–1271.
National Astronomical Observatory of Japan Available at http://eco.mtk.nao.ac.jp/koyomi/index.html.en accessed on 1 November 2011.
Bewick V, Cheek L, Ball J . Statistics review 10: further nonparametric methods. Crit Care 2004; 8: 196–199.
Zhang J, Ma RC, Kong AP, So WY, Li AM, Lam SP, Li SX, Yu MW, Ho CS, Chan MH, Zhang B, Wing YK . Relationship of sleep quantity and quality with 24-hour urinary catecholamines and salivary awakening cortisol in healthy middle-aged adults. Sleep 2011; 34: 225–233.
Scheer FA, Hilton MF, Mantzoros CS, Shea SA . Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009; 106: 4453–4458.
Favero G, Rodella LF, Reiter RJ, Rezzani R . Melatonin and its atheroprotective effects: a review. Mol Cell Endocrinol 2014; 382: 926–937.
Chobanian AV . Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357: 789–796.
Hermida RC, Ayala DE, Fernández JR, Calvo C . Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 2008; 51: 69–76.
Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W . Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol 1999; 55: 111–115.
Nathan PJ, Maguire KP, Burrows GD, Norman TR . The effect of atenolol, a beta1-adrenergic antagonist, on nocturnal plasma melatonin secretion: evidence for a dose–response relationship in humans. J Pineal Res 1997; 23: 131–135.
Brismar K, Mogensen L, Wetterberg L . Depressed melatonin secretion in patients with nightmares due to beta-adrenoceptor blocking drugs. Acta Med Scand 1987; 221: 155–158.
Wikner J, Svanborg E, Wetterberg L, Röjdmark S . Melatonin secretion and excretion in patients with obstructive sleep apnea syndrome. Sleep 1997; 20: 1002–1007.
Hernández C, Abreu J, Abreu P, Castro A, Jiménez A . Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J 2007; 30: 496–500.
The National Health and Nutrition Survey Japan 2010 Available at http://www.mhlw.go.jp/bunya/kenkou/eiyou/h22-houkoku.html Japanese; accessed on 1 November 2011.
Acknowledgements
We thank Sachiko Uemura and Naomi Takenaka for their valuable support during data collection. This work was supported by grants from the Department of Indoor Environmental Medicine, Nara Medical University; Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology; Mitsui Sumitomo Insurance Welfare Foundation; Meiji Yasuda Life Foundation of Health and Welfare; Osaka Gas Group Welfare Foundation; Japan Diabetes Foundation; and Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Obayashi, K., Saeki, K., Tone, N. et al. Relationship between melatonin secretion and nighttime blood pressure in elderly individuals with and without antihypertensive treatment: a cross-sectional study of the HEIJO-KYO cohort. Hypertens Res 37, 908–913 (2014). https://doi.org/10.1038/hr.2014.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2014.99
Keywords
This article is cited by
-
Diminished circadian blood pressure variability in elderly individuals with nuclear cataracts: cross-sectional analysis in the HEIJO-KYO cohort
Hypertension Research (2019)
-
Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice
Hypertension Research (2017)
-
Circadian clock and the onset of cardiovascular events
Hypertension Research (2016)
-
A role for circadian clock in metabolic disease
Hypertension Research (2016)
-
Estimation methods for human circadian phase by use of peripheral tissues
Hypertension Research (2016)