Abstract
Purpose
Laser ‘toys’ can be purchased online and imported with relative ease; the variety of such devices is a potential public safety concern. We describe five children with maculopathy following exposure to laser ‘toys’.
Methods
Case series of maculopathy following exposure to laser ‘toys’.
Results
Five children were seen in our Ophthalmic Unit with macular injuries following exposure to laser ‘toys’. Clinically, three children had an acute vitelliform-like maculopathy which resolved to leave sub-foveal retinal pigment epithelium changes with reduced vision. One case was complicated by a choroidal neovascular membrane.
Conclusion
Laser ‘toys’, which resemble laser pointers, are increasingly available over the internet. Such ‘toys’ may not meet safety standards. Retinal injury in childhood following exposure to laser ‘toys’ is a public safety concern.
Similar content being viewed by others
Introduction
Legislation covers the manufacture and supply of laser products in the European Union and includes inter alia the British Standard on Laser Safety, BS EN 60825-1:2007 (BS EN 60825-1:2007—Safety of Laser Products Pt1: Equipment classification and requirements). Lasers are grouped into ‘classes’ according to their potential for harm. Public Health England (PHE) recommends that so-called toy lasers should be British Standard Class 2 lasers or less (Public Health England website http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733794576). The Food and Drug Administration (FDA) recently alerted consumers about the risk of eye and skin injuries from exposure to high-powered laser pointers. FDA regulations limit the energy output of hand-held laser pointers to 5 milliwatts (mW).1 However, laser ‘toys’, of uncertain safety classification and which resemble low-power laser pointers, can be purchased online from outside Europe and USA. Such lasers have the potential for retinal damage. Importantly as laser technology continues to develop, more powerful portable (hand-held) lasers are being produced at lower cost. We report five local children with maculopathy following exposure to laser toys purchased online and imported to the UK.
Case reports
Case 1
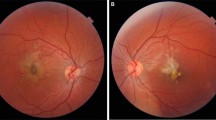
A nine-year-old boy, with a history of right amblyopia, presented on Boxing Day with a 24 h history of painless vision loss in his better eye. He had attended a community optometrist 3 days previously, when his vision was 6/5 in his left eye. At presentation, corrected Snellen vision was 6/12 in the right eye and 6/15 in the left. An acute vitelliform-like maculopathy was present in the left eye (Figures 1a and b), and the right macula was normal. This child initially was commenced on treatment against toxoplasma, with oral steroid cover (20 mg prednisolone/day). Investigations for infective, inflammatory, and paraneoplastic causes all proved negative. Three days later, the vitelliform-like changes resolved to leave RPE changes at the left macula (Figure 1c). The family mentioned that the child had been given a laser ‘toy’ pointer, purchased via the internet, and had been playing with this on Christmas Day. The child denied looking directly into the laser beam. Examination of the ‘toy’ laser pointers bought by the family revealed three separate laser devices made in China, blue (405 nm), green (532 nm), and red (650 nm) with outputs of 57 mW (blue laser), 42 mW (green laser) and 72 mW (red laser) respectively (Figures 2a and b). The British Standard states Class 3R lasers should be <5 mW. At most recent examination, 9 months post presentation, the child’s best corrected visual acuity was 6/9.5 and the OCT imaging reveals persistent outer retinal layer disruption at the fovea.
Case 2
An 11-year-old boy was referred by his community optometrist with recent onset bilateral decreased vision (best-corrected Snellen acuity of 6/7.5 both eyes at baseline) with bilateral ‘yellow’ macular lesions. Retinal photographs taken by the optometrist revealed a bilateral vitelliform-like maculopathy, which had resolved to leave sub-foveal RPE changes when he was seen in the paediatric ophthalmology clinic 8 weeks later (Figures 3a and b). At this point his recorded acuities had deteriorated to 6/12 in the right eye and 6/15 in the left eye. This child admitted that a friend aimed a laser ‘toy’ into both his eyes prior to him developing decreased visual acuity. We were not able to examine the laser device responsible for injury in this case.
Case 3
A 15-year-old girl presented with a 24 h history of blurred vision after shining a laser pointer pen into both eyes for 30 s the previous day. The visual acuity was 6/7.5 in the right eye and 6/6 in the left, although the patient described scotomas in both eyes. Examination revealed a bilateral vitelliform-like maculopathy. This young person has, so far, failed to attend for follow-up after this initial consultation.
Case 4
An 8-year-old boy attended the emergency department following a minor injury. It was noted that his right visual acuity was reduced and a referral to the paediatric ophthalmology service was made. The child was seen the following day. His best-corrected visual acuity was 6/12 in the right eye and 6/6 in the left. There was no history of amblyopia or significant refractive error. Examination revealed retinal pigment epithelial changes at the right fovea, consistent with laser burns. The child admitted to playing with a laser pointer a few months previously, but denied pointing it directly at his eye. It has not been possible to examine the laser that has caused this injury.
Case 5
A 13-year-old boy presented with a 2-month history of decreased vision in his right eye. On direct questioning, he admitted shining a laser pointer into this eye before noticing a visual decline. On examination, the best corrected vision in his right eye was 6/36 and 6/6 in the left. Examination revealed a fibrosed choroidal neovascular membrane at the right fovea and a normal left eye.
Discussion
Laser technology is evolving and laser products are becoming cheaper. So called laser ‘toys’ can be readily purchased online. It may be difficult to discern if such imported laser toys meet relevant safety standards. Our five children developed maculopathy following exposure to these laser devices, three with a vitelliform-like maculopathy in the acute phase. Similar macular disturbance has been reported following exposure to laser pointers in children.2, 3, 4 Furthermore similar changes occurred wherein a patient with an ocular melanoma was exposed to a Class 3A green laser pointer prior to enucleation.5 The retinal damage reported following such injuries is variable.4, 5, 6 This is due to variety of laser powers and wavelengths as well as ocular factors such as fundal pigmentation, blink responses, pupil size, and proximity of the laser burn to the fovea.5 Assessment of alleged laser eye injury requires accurate history and examination.6 Treatment for such laser retinal injuries is uncertain. Oral corticosteroids are sometimes administered.7
The present case series highlights the ocular hazards posed by some laser devices, marketed as ‘toys’. With the expansion of online consumer purchasing the regulation and classification of such laser devices is critical. We are also aware of other children in the UK with retinal injury from imported laser pointers purchased in Asia. Such matters have recently been reported from the Kingdom of Saudi Arabia.8 Furthermore, one of us (SPK) readily purchased a 1.6 Watt hand-held blue laser pointer from a street vendor in China, which would be classified as Class 4 in UK. We wish to raise awareness of this matter as our experience is that children are often reluctant to admit to such mechanisms of injury. Furthermore, consumers and parents need to be alerted to the potential danger such so-called laser ‘toys’ pose to vision. We suggest that children should not be given laser pointers as toys.

References
Food and Drug Administration, http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm237129.htm, 2010.
Dirani A, Chelala E, Fadlallah A, Antonios R, Cherfan G . Bilateral macular injury from a green laser pointer. Clin Ophthalmol 2013; 7: 2127–2130.
Fujinami K, Yokoi T, Hiraoka M, Nishina S, Azuma N . Choroidal neovascularization in a child following laser pointer-induced macular injury. Japan J Ophthalmol 2010; 54 (6): 631–633.
Turkana K, Bryan JS, Gordon AJ, Reddy R, Kwong HM, Sell CH . Laser pointer induced macular damage: case report and mini review. Int Ophthalmol 2012; 32 (3): 293–297.
Robertson DM, McLaren JW, Salomao DR, Link TP . Retinopathy from a green laser pointer: a clinicopathologic study. Arch Ophthalmol 2005; 123 (5): 629–633.
Mainster MA, Stuck BE, Brown J . Assessment of alleged retinal laser injuries. Arch Ophthalmol 2004; 122: 1210–1217.
Barkana Y, Belkin M . Laser eye injuries. Surv Ophthalmol 2000; 44 (6): 459–478.
Alsulaiman SM, Alrushood AA, Almasaud J, Alzaaidi S, Alzahrani Y, Arevalo JF et alKing Khaled Eye Specialist Hospital Collaborative Retina Study Group. High-power handheld blue laser-induced maculopathy: the results of the King Khaled Eye Specialist Hospital Collaborative Retina Study Group. Ophthalmology 2013 e-pub ahead of print 1 November 2013; doi: doi:10.1016/j.ophtha. 2013.09.006.
Acknowledgements
We thank Steven Carley of the Medical Physics Dept who undertook the tests in Sheffield and to Dr Colin Swift, Medical Physics and Engineering Department, The Christie NHS Foundation Trust, Manchester. We also thank Dr John O′Hagan from the Laser and Optical Radiation Dosimetry Group, Public Health England. We would also like to thank Kim Foster, of the Photography Department in Sheffield, for help in preparation of images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Raoof, N., Chan, T., Rogers, N. et al. ‘Toy’ laser macular burns in children. Eye 28, 231–234 (2014). https://doi.org/10.1038/eye.2013.315
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.315
Keywords
This article is cited by
-
Vitrectomy for laser-induced full-thickness macular hole
BMC Ophthalmology (2021)
-
Inner choroidal ischaemia and CNV due to handheld laser-induced maculopathy: a case report and review
Eye (2020)
-
Handheld laser devices and laser-induced retinopathy (LIR) in children: an overview of the literature
Eye (2019)
-
Retinal burns from laser pointers: a risk in children with behavioural problems
Eye (2019)
-
Response to ‘Comment on ‘Toy’ laser macular burns in children: 12-month update’
Eye (2017)